A versatile, compact, and high-performance Breakthrough Analyzer.
- Advanced design reduces dead volume, ensuring precise and reliable experimental outcomes
- Customizable with up to six precision mass flow controllers and two vapor sources
- Enables sample activation up to 1050 ℃
- Equipped with a thermostated environmental chamber for consistent temperature control, even when utilizing vapors
- Utilizes patented high-performance blending valves
- Seamless integration with commercial Mass Spectrometer (MS) and Fourier Transform Infrared Analyzer (FTIR)
- Secure door lock system for enhanced user safety
Description
Engineered for Performance
The Breakthrough Analyzer (BTA) is an adaptable gas delivery and management system designed for the precise characterization of adsorbent performance under process-relevant conditions. This system provides reliable adsorption data for gas/vapor mixtures through a flow-through system.
The BTA is a safe and highly optimized device for collecting transient and equilibrium adsorption data for multi-component systems. It can be customized with up to six precision mass flow controllers and features patented high-performance blending valves, offering unparalleled flexibility in experimental design. The gas-delivery design is superior, allowing precise control over composition and flow rate while minimizing dead volume.
Constructed from high-quality stainless steel, the column of the BTA can accommodate 0.05 to 2.5 g of adsorbent material. Automated sample activation up to 1050 °C is possible with the rugged, precise, and reliable resistance furnace.
Operating pressures are meticulously controlled from atmospheric to 30 bar through a servo-positioned controlled valve.
The thermostated environmental chamber ensures uniform temperature control throughout the entire system, eliminating cold spots and maintaining temperatures up to 200 °C. Operator safety is guaranteed during analysis thanks to the BTA secure door lock system.
For experimental studies, vapor generators can be added to the BTA, facilitating the use of crucial probe molecules such as water. The BTA seamlessly connects to commercially available FTIR and MS systems, enabling efficient gas identification and quantification.
Safety and Features

Image Credit: Micromeritics Instrument Corporation
A safe and finely-tuned apparatus designed for gathering both transient and equilibrium adsorption data in multi-component systems.
- Software-initiated shutdown
- Door closure during analysis
- Alarm for furnace temperature control
- Optional valve for shutting off all Mass Flow Controllers (MFCs)
- Optional integration with shutdown system
- Optional gas detector in the thermostated environmental chamber
- Optional shut-off valve for added control
Temperature-Controlled Thermostated Environmental Chamber
Prevents condensation of vapor streams.
Electropolished 316 SS Sample Column
Capable of holding up to 2.5 g, it is suitable for powder use; alternative diameters are also available for pellets or extrudates.
Touch Screen
Facilitates straightforward operation of the instrument and enables the monitoring of experimental conditions.
Sample Column
It is specifically tailored for handling small sample quantities.
Proprietary Blending Valves
Offer significant benefits in terms of gas mixing and reduction of system dead volume.
Addition of Detectors and Other Optional Accessories
The system's scalability allows for the gradual enhancement of capabilities by integrating detectors and various optional accessories (such as a MS, gas-chromatography-mass spectrometry, additional vapor sources, and vacuum activation, among others available upon request).
Automatic Door Lock
Guarantees temperature stability throughout the analysis process while prioritizing user safety.
Up to 6 Gas Inlets and 2 Vapor Sources
Providing an extensive array of analysis possibilities.
Specifications
Source: Micromeritics Instrument Corporation
| . |
. |
| Furnace Temp Max |
1050 °C |
| Thermostated environmental chamber Temp Max |
200 °C |
| Sample Mass |
Up to 2.5 g |
| Sample Volume |
Up to 2.5 mL |
Additional Specifications
- Determination of breakthrough curves
- Investigation of kinetic performance of adsorbents
- Determination of sorption selectivity
- High-resolution separations using small sample quantities
- Study of co-adsorption and displacement
- Dynamic adsorption and desorption experiments
- Ultra-low dead volume for rapid signal response
- Automated switching between purge and process gases
- Fully automated control via PC
- Up to 6 high-precision mass flow controllers
- Determination of single- and multi-component adsorption data
- In-situ sample preparation up to 450 °C with a stainless steel column and 1050 °C with a quartz column
- Programmable control of total pressure, flow rate, composition, and temperature
- Optimized for research-scale sample sizes with interchangeable reactor beds
- Configurations for gas-vapor and vapor-vapor separation
- Touch Screen interface
- Locked door during analysis to ensure user and analysis protection from altered temperature conditions
- Patented “No Dead Volume” mixing valve with rapid switching
Configurations
Mass spectrometer
In multicomponent adsorption studies, MS is frequently employed to monitor the residual gas composition. The MS is a prevalent detection method utilized for breakthrough analysis.
FTIR Analyzer
FTIR spectrometers are commonly chosen for experimental breakthrough studies, including the separation of xylenes or other aromatic hydrocarbons.
Humidity Sensor
Enables cost-effective direct monitoring of water content, particularly valuable in applications related to production control.
Sample Preparation System
Small quantities of active material can be mixed with an inert carrier to generate a homogenous sample and enhance analysis reproducibility.
CO2 Sensor
Enables cost-effective, direct monitoring of CO2 content, particularly valuable in applications related to production control.
Sample Column (Different Volume)
The BTA is compatible with various column diameters to cater to different sample morphologies, including powders, pellets, and extrudates.
MFC and Mixing Valves (Maximum 6 Gas Inlets)
Supplementary mass flow controllers and blending valves can be incorporated into the BTA to enhance analytical capabilities and broaden the scope of feasible experiments.
Vapor Source (Max of 2)
Optional vapor sources for the BTA are compatible with moisture and other vapors, including xylenes or other aromatics.
Application and Materials
Popular Applications
Direct Air Capture
Differential Absorption Lidar (DAC) poses challenges due to the low concentrations of carbon dioxide (CO2) in the air, coupled with other impurities such as moisture. The captured CO2 can sequestered underground, sold, or converted into value-added chemicals to counterbalance carbon emissions.

Image Credit: Micromeritics Instrument Corporation
CO2 Adsorption
CO2 emissions from power generation, chemical plants, and refineries represent significant point sources, and the elevated concentrations often necessitate distinct operating conditions compared to direct air capture.
Image Credit: Micromeritics Instrument Corporation
Olefin/Paraffin Separations
They constitute a fundamental component of the petrochemical industry and are employed in the manufacturing of polymers such as polyethylene and polypropylene. These separation processes are energy-intensive and contribute to an increase in CO2 emissions.
Image Credit: Micromeritics Instrument Corporation
Natural Gas Separation
Before being utilized in industrial applications and households for heating and food preparation, natural gas, which is a blend of hydrocarbons and various gases, must undergo purification.

Image Credit: Micromeritics Instrument Corporation
Toxic Gas Adsorption
Porous solids find application in personal protection and are currently under development for capturing toxic gases, such as sulfur dioxide, hydrogen sulfide, and nitrogen dioxide, from natural gas or other process feeds.
Image Credit: Micromeritics Instrument Corporation
Water Adsorption
Extraction of water from the air could be a crucial technology in regions with limited access to clean water, either due to arid climates or the escalating demand for water in agriculture.
Image Credit: Micromeritics Instrument Corporation
Materials
Zeolites
Pressure swing adsorption employs Zeolites, such as 5A, 13X, or LiX, which have high selectivity for nitrogen adsorption. This process is utilized in air separation and oxygen production.
Image Credit: Micromeritics Instrument Corporation
Silicas
Amine-functionalized silicas serve as efficient and remarkably selective adsorbents, finding application in the direct air capture of CO2.

Image Credit: Micromeritics Instrument Corporation
Porous Membranes/Monoliths
To enhance the operational efficiency of separation processes, porous membranes, and monoliths coated with zeolites or metal-organic frameworks (MOFs) are frequently employed.

Image Credit: Micromeritics Instrument Corporation
Activated Carbon
Activated carbon-filled canisters are utilized to capture volatile organic components (VOCs) emitted from automobile fuel systems, thereby minimizing these VOC emissions.

Image Credit: Micromeritics Instrument Corporation
Porous Aluminas
Alumina-supported ionic liquids prove to be effective adsorbents with promising applications in the separation of CO2 from natural gas.

Image Credit: Micromeritics Instrument Corporation
Metal-Organic Frameworks
MOFs exhibit high selectivity as adsorbents, making them effective for challenging commercial applications, including the separation of alkanes and olefins, olefins and alkynes, direct air capture, and the separation of CO2 and methane (CH4).
Image Credit: Micromeritics Instrument Corporation
Breakthrough Adsorption Theory
Breakthrough analysis stands as a potent technique for assessing the sorption capacity of an adsorbent under flow conditions. Dynamic breakthrough adsorption offers numerous advantages compared to static adsorption measurements.
- Efficiently gather data on multicomponent adsorption
- Recreate process conditions
- Assess adsorbate selectivity
When performing breakthrough analysis, careful sample preparation is crucial to avoid issues like pressure drop and mass transfer limitations.
Pressure drop occurs when the interstitial space between particles is not large enough to accommodate the gas flow rate, while mass transfer limitations arise when the material's pore size is similar to the kinetic diameter of the adsorbate. Properly sizing particles is thus essential for achieving optimal results.
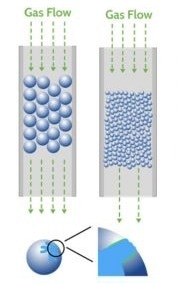
Image Credit: Micromeritics Instrument Corporation
Multicomponent Vapor Analysis
The Micromeritics BTA can concurrently pass two vapor streams through its packed column. Its thermostated environmental chamber prevents condensation during analysis, maintaining a consistent temperature for all gases and vapors within the instrument.
Vapor streams are produced using a bubbler, which enables a carrier gas to reach saturation with the selected vapor. The accompanying figure illustrates multicomponent ethanol/water breakthrough measurements performed on zeolite 13X.
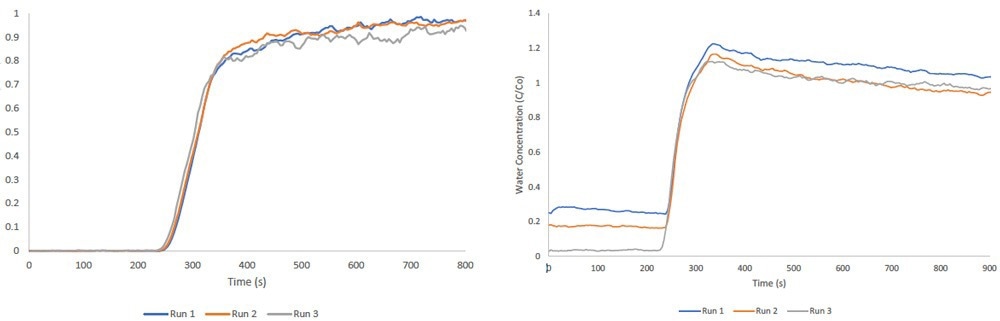
Image Credit: Micromeritics Instrument Corporation
Examining a Breakthrough Curve
- Complete Adsorption
The adsorbate gas is entirely adsorbed, rendering it undetectable at the outlet of the breakthrough column.
- Breakthrough
The adsorbate gas is initially identified at the outlet of the breakthrough column. Gas adsorption persists, however, the adsorbent reaches a point where it can no longer adsorb all of the incoming gas.
- Saturation
The adsorbent has become saturated, reaching a point where it can no longer adsorb the adsorbate gas, enabling the gas to pass through the column unimpeded.
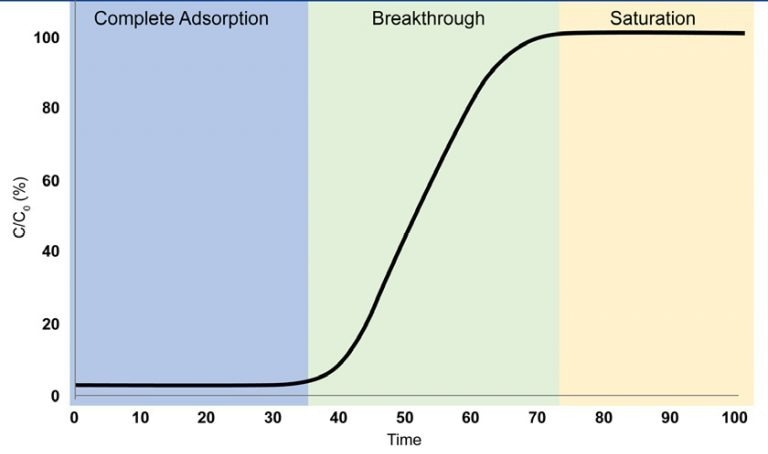
Image Credit: Micromeritics Instrument Corporation
Carbon Dioxide Adsorption
Single-component CO2 breakthrough adsorption experiments were executed using zeolites 13X and 5A, as well as metal-organic frameworks MIL-53(Al) and Fe-BTC. All materials were analyzed at 30 °C with an equimolar gas stream comprising 10 sccm nitrogen and 10 sccm CO2.
A 1 sccm stream of helium was also introduced into the feed gas stream as a tracer gas to assist in determining the onset of the breakthrough experiment.
The breakthrough curves for these materials are depicted below on a mass-normalized axis. The overall quantity of CO2 adsorbed follows this trend: molecular sieve 5A > zeolite 13X > Fe-BTC > MIL-53(Al). The table provided outlines the total quantity adsorbed in mmol/g.
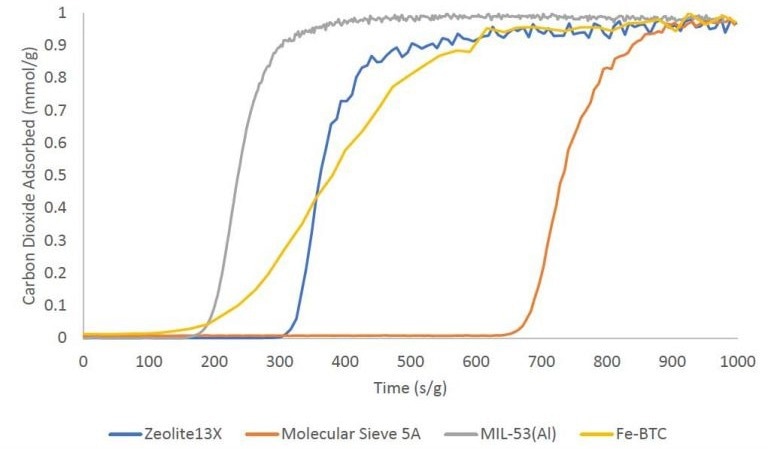
Image Credit: Micromeritics Instrument Corporation
Source: Micromeritics Instrument Corporation
| MATERIAL |
CARBON DIOXIDE ADSORBED (MMOL/G) |
| ZEOLITE 13X |
2.94 |
| MOLECULAR SIEVE 5A |
3.52 |
| MIL-53 (AI) |
1.23 |
| FE-BTC |
2.30 |
High-Pressure Adsorption
The application of zeolite 13X in catalysis and adsorption has been extensively studied. This study employed zeolite 13X as an adsorbent for CO2 adsorption to generate breakthrough curves within the pressure range of 1 to 10 bar.
Equimolar flow rates of 10 sccm nitrogen and 10 sccm CO2 were used for these measurements. A 1 sccm stream of helium served as a tracer gas to identify the start of the breakthrough experiment. All measurements were conducted at an analysis temperature of 30 °C. Between each measurement, the zeolite 13X sample was reactivated overnight to guarantee complete desorption of CO2.
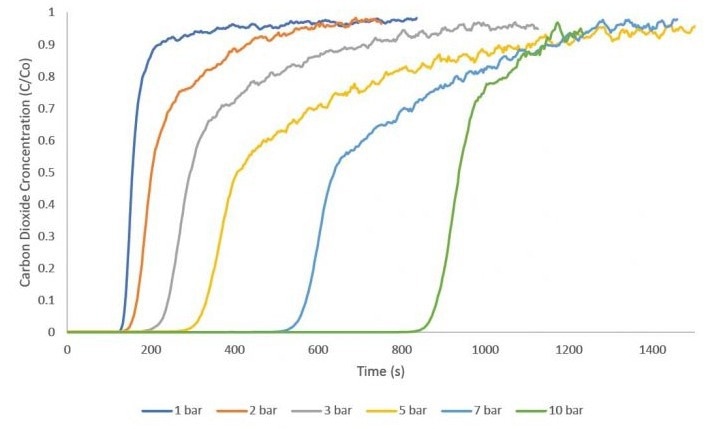
Image Credit: Micromeritics Instrument Corporation
The figure illustrates a consistent increase in breakthrough time across successive experiments as the pressure is elevated. Following the CO2 breakthrough measurements, an equilibrium adsorption quantity was determined for each curve by solving the breakthrough equation.
An isotherm was subsequently constructed, demonstrating the quantity of CO2 adsorbed at 1, 2, 3, 5, 7, and 10 bar total pressure. At 10 bar, zeolite 13X demonstrated an adsorption capacity of approximately 15 mmol/g for CO2.
Although isothermal data collected through breakthrough experiments may not be directly comparable to static adsorption measurements, it offers an evaluation of the adsorbent under conditions relevant to the process.
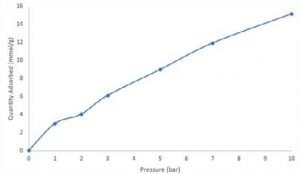
Image Credit: Micromeritics Instrument Corporation
Software
BTA Software
MicroActive is distinguished as the most intuitive, versatile, and comprehensive analysis software designed specifically for adsorption studies. Its adaptability, user-friendly interface, and ease of operation make it suitable for a wide range of experimental conditions and automates the breakthrough process from sample activation to analysis, such as the execution of cyclic experiments.
Image Credit: Micromeritics Instrument Corporation
When integrated with the industry-leading MicroActive analysis software, the BTA system precisely and reproducibly characterizes adsorbents.
Utilizing comprehensive analysis methods, it effectively analyzes data and adeptly solves the breakthrough equation, even for the most challenging samples.
MicroActive Software enables
- Data reduction from MS
- Quantity adsorbed selectivity
Micromeritics BreakThrough Analyzer - Product Overview
Video Credit: Micromeritics Instrument Corporation