Size-exclusion chromatography (SEC) or gel permeation chromatography (GPC) is an extensively used method for characterizing a wide range of macromolecules, which includes but is not limited to bulk or commodity manufactured polymers. This method can be used for measuring the molecular weight moments, the intrinsic viscosity, molecular weight distribution and/or hydrodynamic size of these macromolecules. A complete GPC system setup is shown in Figure 1.

Figure 1. Malvern Panalytical Viscotek TDA Triple Detection GPC System.
While both practical and effective conditions have been determined for most of these commodity polymers such as chlorinated benzenes for polyolefins, THF for polystyrene, some need further optimization in one aspect or another. This article describes one such polymer, nylon, where operating conditions changes can result in improvements in health, cost and safety.
GPC Analysis of Nylon Traditional/Historical Analysis of Nylon
Typical GPC analysis of nylons involves using phenolic based solvents (o-chlorophenol, m-cresol, phenol blends) or hexafluoroisopropanol (HFIP) for the dissolution solvent and/or mobile phase. Out of these solvents, HFIP offers considerable benefits over the phenolic solvents with lower quantifiable health and safety hazards and considerably higher signal-to-noise ratios in both light scattering and refractive index detectors because of a higher dn/dc. The key disadvantage of HFIP is the prohibitive cost ranging from $2 to $4 (or more) per mL.
Formic acid has been used historically for determining the solution viscosity of nylon. From previously mentioned metrics, formic acid is as good as or better than HFIP in terms of safety and health. The LD50 limit is a bit higher with formic acid and the considerably low pressure of formic acid results in a lower potential for inhalation exposure. In terms of price, formic acid costs considerably less than HFIP, in the range of $0.15 to $0.30 per mL.
Certain drawbacks to the use of formic acid as a mobile phase are that it does not dissolve all forms of nylon, especially nylon-11 and nylon-12. Formic acid may also be highly caustic to most traditional components of liquid chromatography systems.
The Viscotek TDA Triple Detection GPC System, shown in Figure 1 and used for this study, has specific, proprietary design components that enable the use of formic acid as a mobile phase. Furthermore, dissolved nylon samples will have a reduced signal-to-noise ration in comparison with HFIP because of a lower dn/dc of these samples in formic acid. It is shown in the article whether or not reduced signal-to-noise will avoid adequate GPC analysis of nylon in formic acid.
GPC Results
A sequence of four nylon- 6 samples were prepared two times for GPC analysis once for analysis in a mobile HFIP phase of 0.05M potassium trifluoroacetate and once for analysis in 88% formic acid. A triple detector chromatogram of one HFIP sample is shown in Figure 2 and a triple detector chromatogram of the same formic acid sample is shown in Figure 3.
These chromatograms show the refractive index signal in red, the right angle light scattering (RALS) signal in green and the viscometer signal in blue. The molecular weight determined at each retention volume is also shown in black to determine the sample's molecular weight distribution.
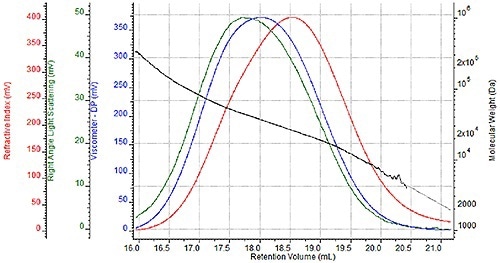
Figure 2. Triple detector chromatogram of nylon-6 sample in HFIP.
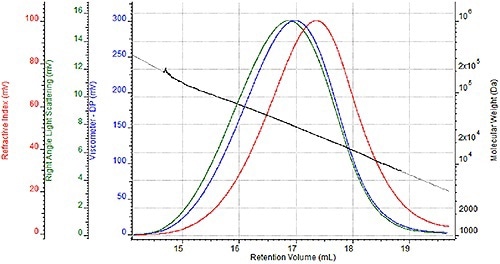
Figure 3. Triple detector chromatogram of nylon-6 sample in 88% formic acid.
While the associated molecular weight distributions and chromatograms appear almost the same with two unique mobile phases, a more comprehensive comparison is required to precisely assess the efficacy of formic acid as a mobile phase.
Table 1 is a comparison of the molecular data obtained for the four nylon-6 samples using HFIP as the mobile phase and using 88% formic acid (FA) as the mobile phase. Presented values include molecular weight distribution moments (Mn, Mw, and Mz) and intrinsic viscosity ([η]). The average of the three injections are shown in the data.
Table 1. Molecular data derived for four nylon-6 samples in HFIP and 88% formic acid.
| Sample ID |
Solvent |
Mn (Da) |
Mw (Da) |
Mz (Da) |
PDI |
[η] (dL/g) |
dn/dc (mL/g) |
| Nylon – A |
HFIP |
21636 |
32651 |
45728 |
1.51 |
1.025 |
0.27 |
| Nylon – A |
FA |
23083 |
32487 |
46085 |
1.41 |
0.970 |
0.12 |
| Nylon – B |
HFIP |
21054 |
30882 |
42775 |
1.47 |
0.979 |
0.27 |
| Nylon – B |
FA |
21471 |
31523 |
46183 |
1.47 |
0.934 |
0.12 |
| Nylon – C |
HFIP |
54449 |
75578 |
103602 |
1.39 |
1.867 |
0.27 |
| Nylon – C |
FA |
49276 |
69940 |
100235 |
1.40 |
1.909 |
0.12 |
| Nylon – D |
HFIP |
41981 |
59200 |
81740 |
1.41 |
1.590 |
0.27 |
| Nylon – D |
FA |
38711 |
54576 |
79453 |
1.41 |
1.559 |
0.12 |
In Table 1, the calculated data for various nylon samples in each mobile phase is shown. The molecular weight distribution is almost identical for all samples less than 10% difference. η also smoothly correlates between the two mobile phases. Here, correlation is almost one though this is more of a coincidence. Different mobile phases may swell the polymer chains to different degrees leading to different [η] for the same sample.
Conclusions
For the GPC analysis if nylon -6,6 and nylon – 6 or other soluble polyamides, formic acid offers a less costly, effective and safe choice to mobile phases conventionally used for these polymers. The Viscotek TDA Triple Detection GPC System offers excellent signal-to-noise ratios for accurate and robust analysis of nylons in formic acid. Furthermore the Malvern Panalytical GPC system has been designed to consistently operate under the potentially corrosive conditions posed by formic acid and other solvents.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.