Differential Scanning Calorimetry (DSC) is a method used for measuring the heat flow and temperature related to thermal changes in materials.
The optical DSC450 system has been improved for users who prefer to quantify the enthalpy changes and transition temperatures of their samples. The design enables mounting the stage on a microscope, thus making it easy to record images and time-lapse of sample transitions at excellent resolution.
With the optical DSC450, users can quantify glass and thermal transitions of an extensive range of substances while precisely regulating temperatures between −150°C and 450°C. The stage contains gas ports that help purge the stage to reduce sample oxidation.
The system is supplied with LINK software, a T96 controller and optional Digital Imaging and Thermal Analysis by Structural Characterization (TASC) modules.
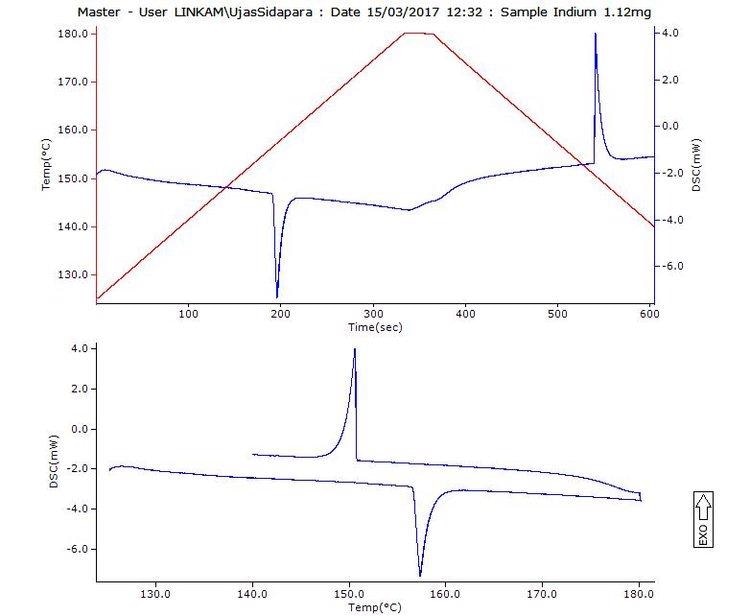
Image Credit: Linkam Scientific Instruments
The image shown above is a standard DSC curve output from the LINK control software. The images given below are of a sucrose grain before melting (left) and during melting (right). The DSC system has an optical feature that enables both data and image sampling all through the experiment.
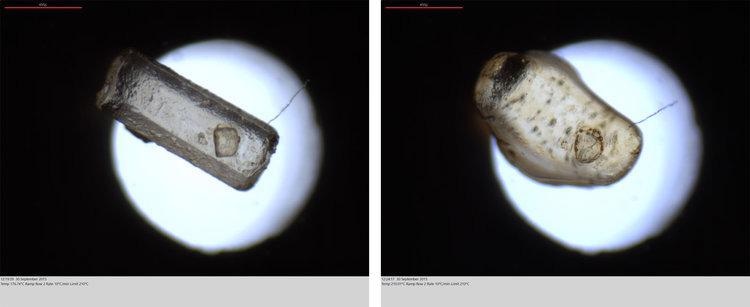
Image Credit: Linkam Scientific Instruments

DSC450 on Imaging Station. Image Credit: Linkam Scientific Instruments
How To Prepare Your DSC Samples
Video Credit: Linkam Scientific Instruments