MIRAP is a renowned handheld Raman system for on-site raw material verification: simple, quick, versatile, and transparent.
Highlights
MIRA P is a huge improvement over conventional raw material identification and verification (RMID) methods, intended for lab-quality results in non-traditional testing scenarios such as materials inspection at the loading dock.
MIRA P rapidly and effortlessly validates the quality and consistency of materials, allowing manufacturers to save time and resources. Metrohm Raman streamlines the process, from method development to model verification and on-the-spot material sampling.
- Clear “Pass” or “Fail” results are provided in seconds.
- Through-package sampling attachments for liquids, solids, and powders are available.
- MIRA Cal P is a dedicated, user-friendly software that supports full compliance for regulated industries.
- Workflows for simple material inspection that are short, simple, and automated.
- Orbital Raster Scan for sensitive interrogation that does not consume, damage, or burn the sample.
Benefits
Fast Results in Seconds

Image Credit: Metrohm AG
MIRA P gathers and processes data, performs statistical analyses, and returns a clear “Pass” or “Fail” result in seconds.
The instrument computes the result using multivariate probabilistic algorithms to confirm the identity of raw materials. This method is far more precise and dependable than conventional HQI spectral matching.
Safe Sampling of Sensitive Materials
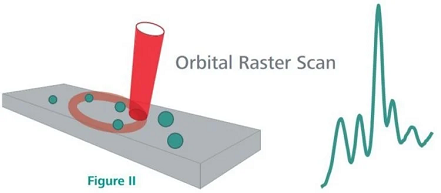
Image Credit: Metrohm AG
Orbital Raster Scan (ORS) is a distinctive characteristic of all Metrohm Raman systems. ORS rasters the sampling laser over a large area to increase the information gathered with each scan. ORS achieves excellent resolution, even in heterogeneous samples, and decreases the likelihood of sample degradation or burning.
Move Sampling from the Lab to the Loading Dock

Image Credit: Metrohm AG
The use of handheld Raman in RMID, where many materials are tested in the receiving area, represents a significant departure from traditional methods.
Non-technical users can also benefit from automated workflows. Anyone can use this to analyze a sample quickly, safely, and accurately.
MIRA Cal P: Dedicated Software for Full Confidence in Compliance
Metrohm Raman is dedicated to providing the easiest, most effective, and most precise use of MIRA P for RMID with MIRA Cal P. The software includes features like complete reports and audit trail functions that ensure secure and well-documented procedures in accordance with FDA 21 CFR Part 11 regulations.
The new Calibrate/Verify Accessory now performs a two-part system verification. The ASTM method E1840 for Toluene-Acetonitrile is used to obtain the best wavenumber calibration possible across the entire range. Second, NIST-traceable polystyrene ensures wavenumber accuracy and compliance with USP 1120 and EP 2.2.48.
Systems
MIRA P Basic

Image Credit: Metrohm AG
MIRA P Advanced

Image Credit: Metrohm AG
MIRA P Flex

Image Credit: Metrohm AG
Options and Upgrades
Sampling
Easier Sampling With Smart Tips
MIRA P can be outfitted with various Smart Tips, which trigger automated instrument routines through container, direct-contact immersion, and controlled sampling.
Software
Compliant Software
The MIRA Cal P software included with the MIRA P instrument is FDA 21 CFR Part 11 compliant. The software includes the following features:
- Access control on multiple levels, with three predefined levels and optional password aging and complexity requirements.
- Audit trails are used to record all instrument actions.
- Secure electronic records that are aligned to a PC-based secure database
- At the press of a button, reports comprising all necessary information (instrument information, parameters, and electronic signatures) are produced.
Mira P—Easy, Fast, Flexible, and Reliable
Flexible: Customize Mira P to Suit Unique Needs
Image Credit: Metrohm AG
- Reports that can be customized
- Operating procedures that can be customized
- A wide range of sampling options
Results That Can be Trusted: Decisions Made With Confidence

Image Credit: Metrohm AG
- Customizable model building
- Discriminatory algorithms
- Clear results
Evaluation Type
- Material testing with Pass/Fail results
- Spectral library searching for material identification
- Mixture matching for multi-component identification
Simple to Use: Straightforward Guided Workflow

Image Credit: Metrohm AG
- Controlled user interface ensures error-free operation.
- Automated report generation
- The transition between samples is seamless.
Barcode Scanning
A barcode scan automatically selects and populates an operating procedure.
Acquisition Parameters
- Integration time
- Smart tips
- Laser power
- Spectral averaging
Fast: Results in Seconds
- Increase throughput
- Complete analysis in seconds
- Move fastly from sample to sample

Image Credit: Metrohm AG
Smart Sampling Attachments for a Wide Range of Different Sample
Unique Flexibility Meets Needs

Image Credit: Metrohm AG
Mira P’s unique set of sampling attachments enables users to ascertain the contents of any container.
Point-and-Shoot Attachments

Image Credit: Metrohm AG
Mira P includes two different point-of-contact sampling attachments. The SWD is intended for direct contact or thin bags, whereas the LWD is intended for thicker containers like glass bottles.
Contact Ball Probe

Image Credit: Metrohm AG
Simply immerse the Contact Ball Probe in a liquid or powder to test materials.
Types and Enhanced User Safety
Tablet Holder

Image Credit: Metrohm AG
Large and small tablets are held in place by a spring-loaded mechanism for final formulation analysis.
Vial Holder

Image Credit: Metrohm AG
The vial holder attachment lets the user quickly and conveniently measure liquid or powdered samples stored in vials.
Calibrate/Verify Accessory (CVA)

Image Credit: Metrohm AG
The CVA is made up of an ASTM Raman shift standard and a NIST-traceable verification sample that adheres to USP/EP guidelines.
Mira P—Fully Compliant With FDA 21CFR Part 11
Mira P complies with FDA 21 CFR Part 11 regulations. It has innumerable security features that go above and beyond regulatory requirements.
- Multilevel access control with individual user login credentials
- There are three predefined access levels: Administrator, Laboratory Manager, and Routine User.
- Password aging and complexity requirements are optional.
- Each measurement on the instrument generates secure electronic records.
- Records can be easily transferred to a secure database.
- Every action performed on the instrument is recorded in the audit trail, including the user, date, time, and sampling parameters.
- Complies with the most recent USP and EP Raman Spectroscopy guidelines.
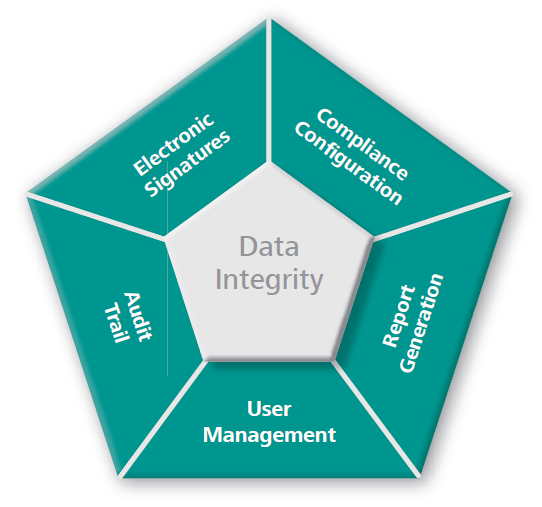
Image Credit: Metrohm AG
Patented ORS Technology—Superior Reproducibility When Measuring Heterogeneous Formulations
Traditional Raman spectrometers use a tightly focused laser beam to achieve high spectral resolution (Figure I). However, because of the small beam diameter and small particle size of several APIs, components in heterogeneous samples can be completely missed. For an accurate, reproducible result, several spectra must be collected at different points on the sample.
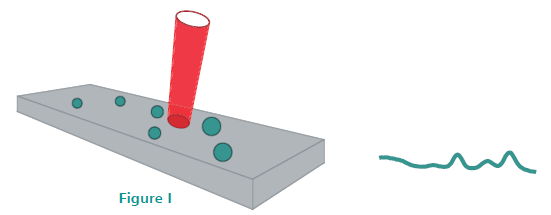
Image Credit: Metrohm AG
The Mira P employs ORS (Orbital Raster Scan) technology, which analyzes a larger sample area and is more likely to capture dispersed sample components (Figure II). Using ORS technology, Mira P captures APIs in heterogeneous formulations in a single analysis.
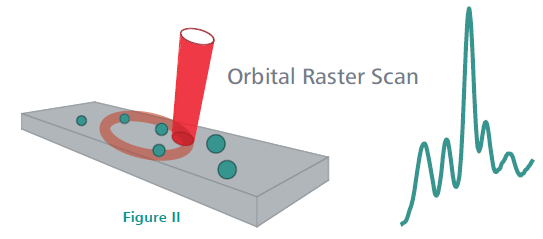
Image Credit: Metrohm AG
True Single-Handed Operation—Just 13.0 cm (h) × 8.5 cm (w) × 4.0 cm (d)
Scale 1:1,8., Mira P handheld Raman Spectrometer. Image Credit: Metrohm AG