A research team from UC Davis and Rice University has discovered a technique that would enable natural proteins to self-assemble into amyloid fibrils. Nature has several instances of self-assembly, and researchers have always been interested in replicating or manipulating these proteins to develop novel and practical materials or devices. The team’s research findings have been published online by the journal ACS Nano.
 Amyloid fibers self-assemble from smaller proteins. UC Davis researchers have engineered other proteins so they spontaneously form amyloid. These new proteins could be useful in nanotechnology. (Credit: UC Davis)
Amyloid fibers self-assemble from smaller proteins. UC Davis researchers have engineered other proteins so they spontaneously form amyloid. These new proteins could be useful in nanotechnology. (Credit: UC Davis)
The amyloid proteins are capable of self-assembling into tangled plaques which are mostly connected with Alzheimer's disease. Similar proteins can be formed into extremely useful materials e.g., spider silk, or biofilms surrounding living cells.
These proteins can be applied as "scaffolding" for tissue engineering, and possess the capability to be programmed in such a manner that other proteins or particles can be fixed in exact arrays or locations. Amyloids are known to be strong with the capability to withstand UV radiation, boiling and attack by digestive proteins.
These are big proteins with lots of flat surfaces suitable for functionalization, for example to grow photovoltaics or to attach to other surfaces.
Dan Cox, a physics professor at UC Davis and coauthor on the paper.
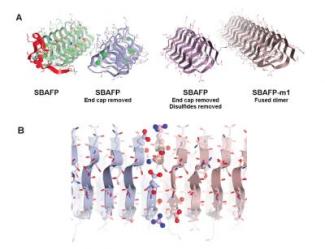
The research team was able to create the amyloid fibrils by modifying the natural "antifreeze" proteins procured from a spruce budworm (insect) and ryegrass. These amyloid proteins allow certain animals and plants to handle extremely cold temperatures by stopping ice crystals from growing on them, however the proteins cannot naturally self-assemble into bigger formations. The researchers were able to eliminate cap formations from the ends of the antifreeze proteins. Then they allowed the proteins to self-assemble into fibrils with certain heights, thereby making them a probable novel material for bioengineering.
References