Feb 7 2017
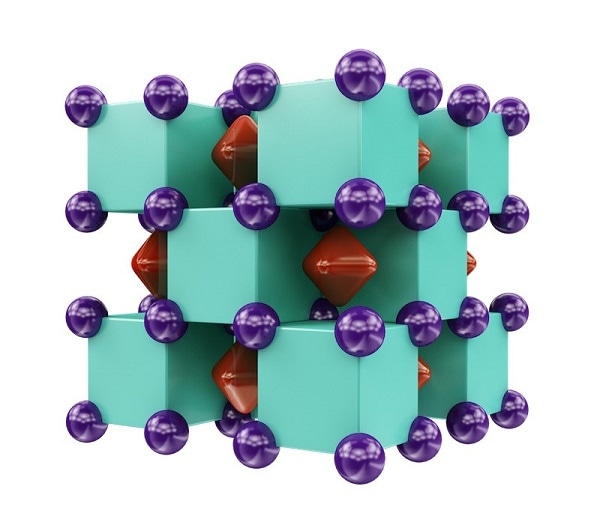 Crystal structure of Na2He, resembling a three-dimensional checkerboard. The purple spheres represent sodium atoms, which are inside the green cubes that represent helium atoms. The red regions inside voids of the structure show areas where localized electron pairs reside. CREDIT: Illustration is provided courtesy of Artem R. Oganov.
Crystal structure of Na2He, resembling a three-dimensional checkerboard. The purple spheres represent sodium atoms, which are inside the green cubes that represent helium atoms. The red regions inside voids of the structure show areas where localized electron pairs reside. CREDIT: Illustration is provided courtesy of Artem R. Oganov.
Despite the fact that helium is the second most abundant element in the universe, (after hydrogen) it does not play well with other elements. It is a member of a family of seven elements known as noble gases. The elements get their name due to their chemical inertness, i.e. the elements do not combine easily with other elements to form compounds. Broadly considered to be the most inert element, helium does not form any stable compounds under normal conditions.
Skoltech’s Prof. Artem R. Oganov, who is also head of Computational Materials Discovery laboratory at Moscow Institute of Physics and Technology and a professor at Stony Brook University, has headed an international team of researchers who have predicted the formation of two stable helium compounds, namely, Na2He and Na2HeO.
The research team provided a theoretical explanation and experimental confirmation of the stability of Na2He. The study could provide valuable insights into the chemistry that take place inside gas giant planets and possibly even stars, where helium is the main component. The study has been published in the Nature Chemistry journal.
The authors of this study used a crystal structure-predicting tool, the first-principles evolutionary algorithm named USPEX, to perform a systematic search for stable helium compounds. This way, they could predict the existence of Na2He. Then, they successfully synthesized Na2He in a diamond anvil cell (DAC) experiment carried out at the Carnegie Institution for Science in Washington.
The DAC experiment was performed by Prof. Alexander F. Goncharov and his collaborators. The compound was formed at pressures equal to approximately 1.1 million times that of Earth’s atmospheric pressure and is estimated to be stable not less than 10 million times than that.
The compound that we discovered is very peculiar: helium atoms do not actually form any chemical bonds, yet their presence fundamentally changes chemical interactions between sodium atoms, forces electrons to localize inside cubic voids of the structure and makes this material insulating.
Xiao Dong
Xiao Dong was a long-term visiting student in Oganov’s laboratory when this research was carried out.
Na2He is an electride - a special type of ionic salt-like crystal - with a positively charged sublattice formed of sodium ions and a negatively charged sublattice formed of localized electron pairs. The strong localization of the electrons in this crystal renders it as an insulator - it does not have the ability to conduct free-flowing electrons in an electric current.
Na2HeO, the other predicted helium compound, was discovered to be stable at a pressure of 0.15 - 1.1 million atmospheres. Na2HeO is also an ionic crystal with its structure similar to that of Na2He, but the electron pairs are replaced by negatively charged oxygen of the form O2-.
This study shows how new surprising phenomena can be discovered by combination of powerful theoretical methods and state-of-the-art experiments. It shows that very weird chemical phenomena and compounds can emerge at extreme conditions, and the role of such phenomena inside planets needs to be explored.
Professor Artem R. Oganov