Developing innovative ways to generate hydrogen is attracting the attention of researchers including a group of materials scientists in Beijing, China who has recently published a paper in the Journal of Magnesium and Alloys. In the paper, the group discusses how they have developed a novel nonporous magnesium-lithium (Mg-Li) material that possesses hydrogen generation properties.

Study: A novel nanoporous Mg-Li material for efficient hydrogen generation. Image Credit: petrmalinak/Shutterstock.com
Many believe that the next step of the global energy transition will be based on the hydrogen economy. Hydrogen fuel can be considered a zero-carbon fuel provided it is produced in a zero-carbon way. Hydrogen is also an energy carrier that can be used to store, move, and deliver energy produced from other sources.
“The improved hydrogen generation property is attributed to the nanoporous structure with a high specific surface area, and the addition of lithium element which acts as active sites in hydrogen generation process,” explains co-author of a study in the Journal of Magnesium and Alloys, Professor Xiping Song of the State Key Laboratory for Advanced Metals and Materials, University of Science and Technology Beijing.
Developing green energy systems to replace traditional fossil fuels addresses some of the concerns climate scientists have raised with regard to the current climate emergency. Green or renewable hydrogen is seen as indispensable in the move towards climate neutrality and many of the goals nations have set for both 2030 (55% reduction in greenhouse gas emissions) and 2050 (net-zero emissions).
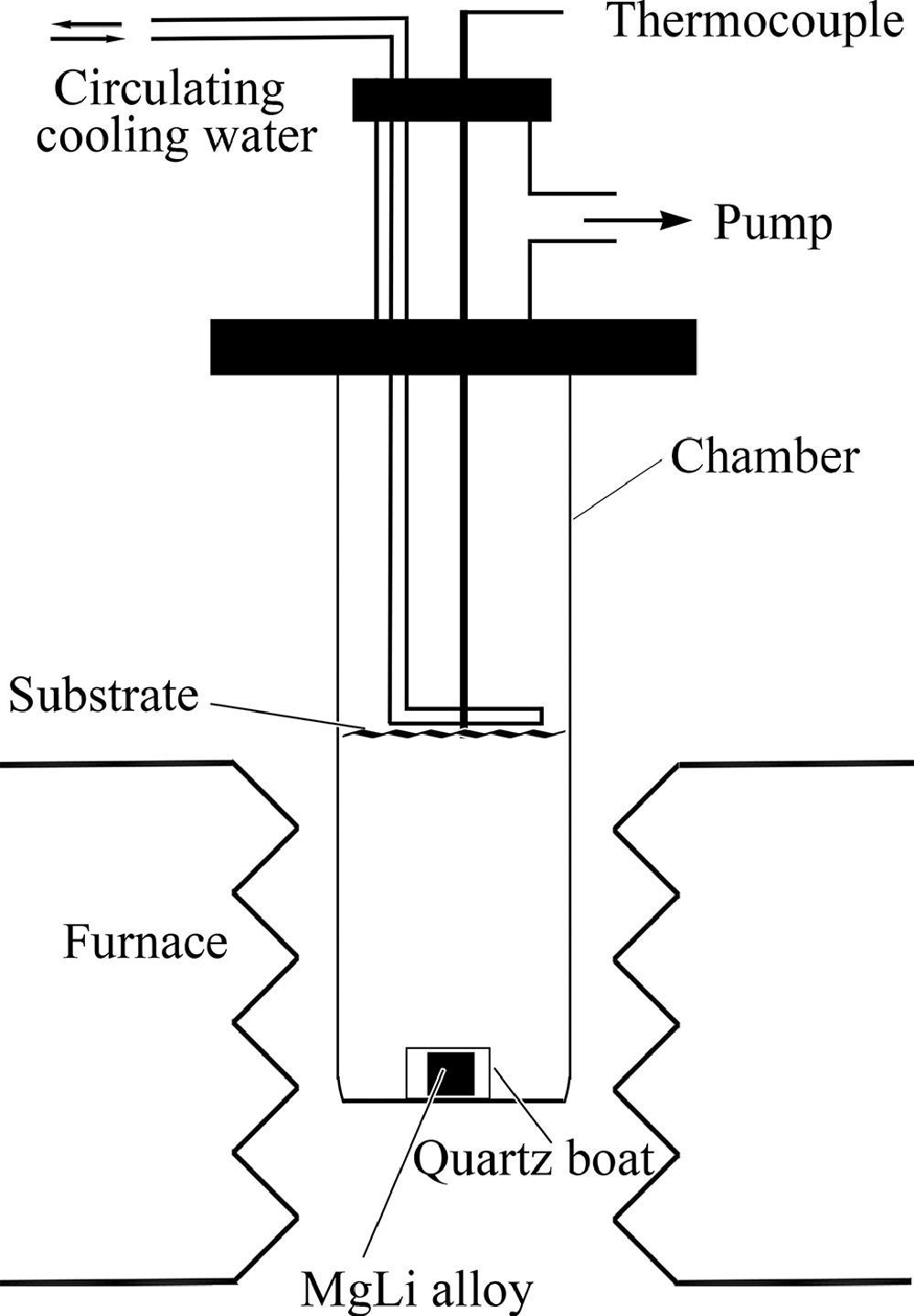
Schematic diagram for the preparation of the nanoporous Mg-Li material. Image Credit: J. Liu et al., Journal of Magnesium and Alloys
Hydrogen Generation
Current hydrogen generation methods include the breaking down of fossil fuels, electrolysis/photolysis of water, and biohydrogen production. Breaking down, or the decomposition of fossil fuels including pyrolysis of petroleum is used extensively throughout industry, but the gas products usually contain impurity gasses, and it necessitates complex and expensive purification process to generate high-purity hydrogen.
Additionally, electrolysis and photolysis require vast amounts of energy and expensive catalysts which can also trickle down and have an environmental impact, while biohydrogen generation is a technical challenge that can be time- and labor-intensive. Moreover, any hydrogen generated using these methods still needs to be stored and transported which in itself can be a costly endeavor.
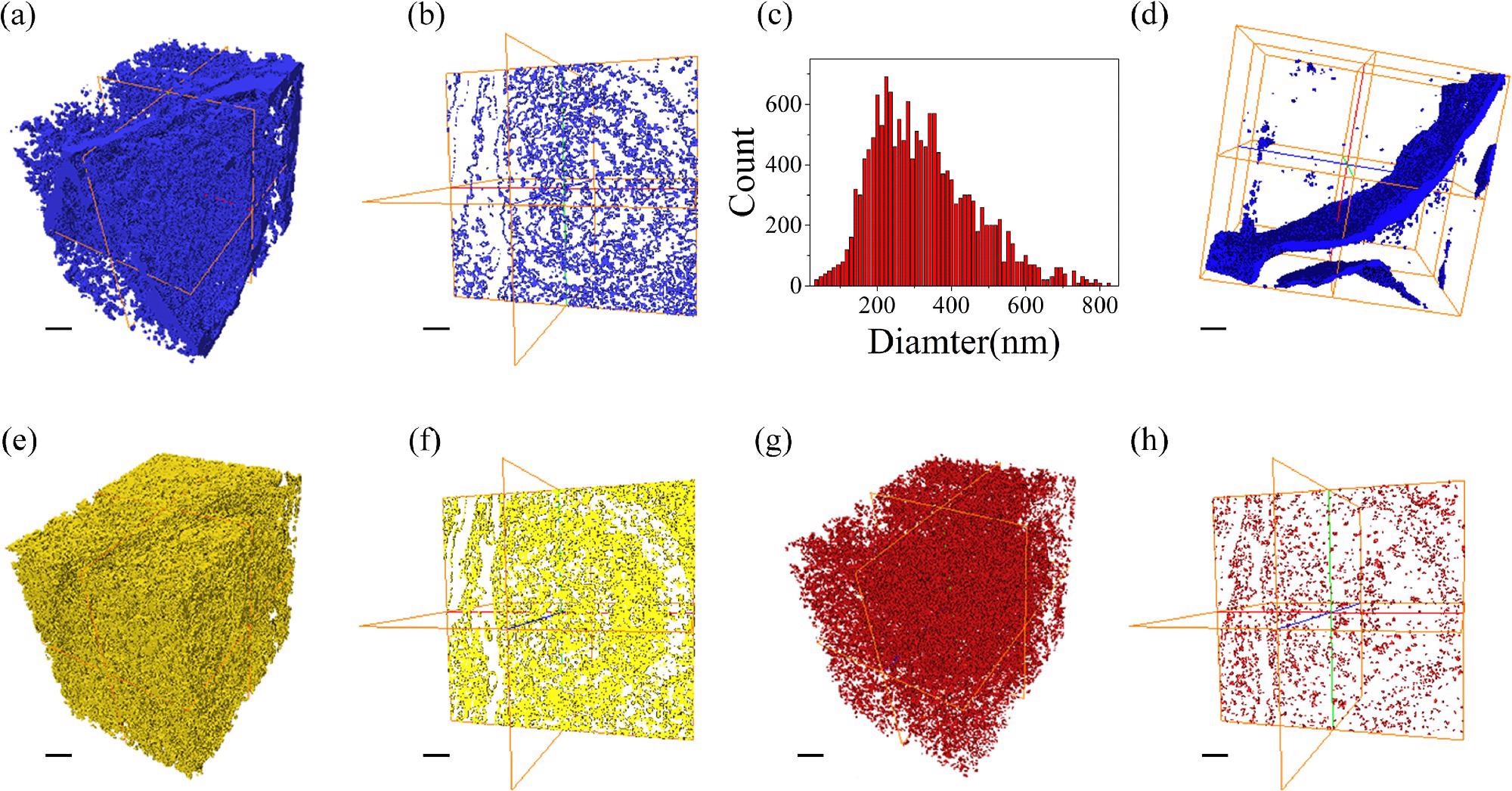
Three-dimensional images of nanoporous Mg-Li materials. (a) the three-dimensional distribution of nanopores and gaps, (b) the cross-section image drawn from (a), (c) frequency of nanopore size, (d) the three-dimensional image of the gaps, (e) the three-dimensional distribution of the Mg atoms, (f) the cross-section image drawn from (e), (g) the three-dimensional distribution of Li atoms, (h) the cross-section image drawn from (g), the line represent 1 μm. Image Credit: J. Liu et al., Journal of Magnesium and Alloys
The researchers stepped in and identified that previous studies have shown there are materials with the capacity to produce hydrogen: “Some hydrogen generation materials can directly produce hydrogen by hydrolysis reaction with water and have many advantages, such as on-site and rapid hydrogen supply, convenient storage and carrying, simple operation and high hydrogen purity,” says Song.
Nanoporous Mg-Li
Song and his team evaluated several materials and noted that magnesium was suitable material for hydrogen generation as it is abundant, cheap, and easy to be recycled. However, during the hydrolysis reaction magnesium produces a byproduct, which is the magnesium hydroxide which forms a deposit that can impede or even stop the reaction and hydrogen production.
By adding lithium to the magnesium, the team was able to improve the hydrolysis reaction and reduce the production of any unwanted byproducts. Yet, the team still needed to develop an effective method to produce the Mg-Li material as traditional ball-milling presents issues including an agglomeration problem making it difficult to produce homogenous Mg-Li material with a high specific surface area.
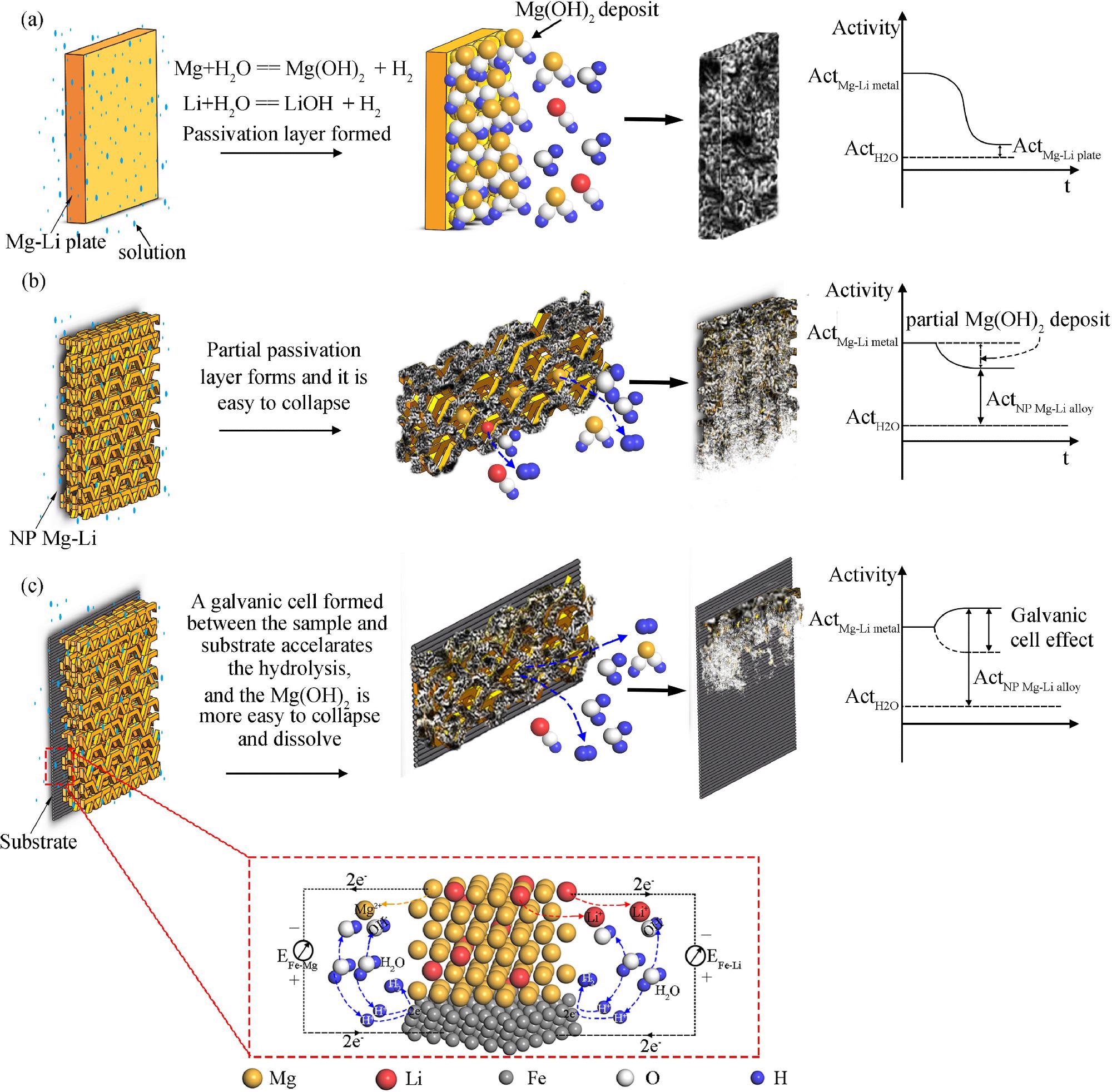
The hydrogen generation mechanisms for three different Mg-Li alloys: (a) the as-received Mg-Li alloy plate, (b) the nanoporous Mg-Li material, (c) the nanoporous Mg-Li material with substrate. Image Credit: J. Liu et al., Journal of Magnesium and Alloys
Thus, the researchers were able to successfully develop and use a simple vapor deposition method for the preparation of a Mg-Li nanoporous material. The nanoporous structure increases the surface area of the material and offers multiple channels for the hydrolysis reaction while the addition of lithium breaks down the magnesium hydroxide deposit.
Energy Transition Potential
The capacity of the Mg-Li material and its effectiveness for hydrogen generation was determined by a saltwater immersion test. “The nanoporous Mg-Li material has an excellent hydrogen generation property, and the preparation is simple, quick, and cost- effective,” says Song.
One of the remarkable things that the team was able to observe was that the amount of hydrogen generated by the nanoporous Mg-Li material reaches over 99% theoretical hydrogen generation amount in the temperature range from 0 °C to 50 °C. Not only does the addition of lithium prevent the byproduct of the hydrolysis reaction from forming, but it also increases the hydrogen generation rate and improves the amount by lowering the density of the material.
With these findings, the team has demonstrated the potential for an innovative hydrogen generation method using a novel nanoporous Mg-Li material that could revolutionize the hydrogen economy in taking the next step in the global energy transition. The implications for future green energy solutions could also help industry and governments in their ambition to meet targets for the reduction of greenhouse gas emissions in the near future.
References
J. Liu, Q. Yuan, W. Huang et al., A novel nanoporous Mg-Li material for efficient hydrogen generation, Journal of Magnesium and Alloys, https://www.sciencedirect.com/science/article/pii/S2213956721002929
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.