While the wheel does not need to be reinvented, the development of new nano-wheels has its advantages, according to a multi-institute research team located in China. The researchers created a new family of metallic compounds, each with distinct features useful for next-generation technology, such as improved sensors.
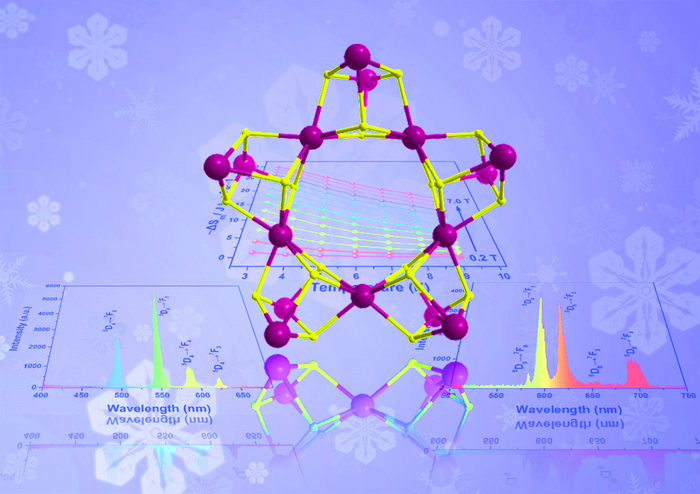
A multi-institute research team synthesized a family of nano-wheel-like metallic clusters, each with specific properties—such as fluorescence and different types of magnetism—that could advance next-generation technologies. Image Credit: Polyoxometalates, Tsinghua University Press.
On March 12th, 2023, the findings were published in Polyoxometalates.
Polymetallic complexes are of great interest not only for their appealing molecular structure but also for their versatile applications in various fields.
Yan-Zhen Zheng, Study Co-Corresponding Author and Professor, Frontier Institute of Science and Technology, Xi’an Jiaotong University
If polymetallic complexes, which are made up of several atoms of different metals or a combination of metals and other elements, can be produced, they have the potential to imbue materials with unique qualities, according to Zheng. The capacity to fluoresce, or glow, and magnetic quirks that allow for extreme temperature swings and control are examples of such capabilities.
Zheng and his colleagues concentrated on the development of polymetallic complexes based on lanthanide elements, a group of 15 metallic compounds commonly known as rare earth elements. They specifically employed europium, terbium, and gadolinium.
Among all polymetallic complexes, lanthanide-based compounds have drawn unprecedented attention due to their interesting magnetic and luminescence behaviors. Several such compounds have been successfully isolated, but direct synthesis has been a challenge.
Yan-Zhen Zheng, Study Co-Corresponding Author and Professor, Frontier Institute of Science and Technology, Xi’an Jiaotong University
According to Zheng, the complexes' components are geometrically diverse, requiring significant coordination.
Previous findings revealed that controlling the hydrolysis—breaking down a compound with water—of lanthanide metal ions in the presence of appropriate organic ligands would be a powerful strategy to obtain desired species.
Yan-Zhen Zheng, Study Co-Corresponding Author and Professor, Frontier Institute of Science and Technology, Xi’an Jiaotong University
A ligand is a molecule that forms a connection with a metal atom. Its inclusion in the complex can help to stabilize the structure.
The researchers used hydrolysis to breakdown lanthanides in a bath containing a ligand called tricine. Tricine contains multiple arms of oxygen and hydrogen, which can accommodate a large range of metals and help stabilize the resulting clusters.
Zheng adds, “Through the simple hydrolysis reaction, we synthesized three lanthanide nano-clusters, and used X-Ray diffraction analyses to reveal their stable, wheel-like structure. Owing to the presence of different lanthanide metal ions in these analogs, each compound shows distinctive properties.”
The europium-based cluster fluoresced red emissions, while the terbium-based cluster fluoresced green emissions. The gadolinium-based cluster showed promise for magnetic cooling applications. According to Zheng, the team is still looking into the synthesis and applications of these clusters.
Peng-Fei Sun, Xiao-Nan Zhang, Cai-Hong Fan, and co-corresponding author Wei-Peng Chen, all with FIST, the State Key Laboratory of Mechanical Behavior for Materials, the MOE Key Laboratory for Nonequilibrium Synthesis of Condensed Matter, the Xi’an Key Laboratory of Sustainable Energy and Materials Chemistry and the School of Chemistry at Xi’an Jiaotong University contributed to the study.
The research was supported by the National Science Foundation of China, the Special Support Plan of Shaanxi Province for Young Top-Notch Talent, the Instrument Analysis Center of Xi’an Jiaotong University, and the Fundamental Research Fund for Central Universities.
Journal Reference:
Sun, P.-F., et al. (2023). Tricine-supported polyoxo(alkoxo)lanthanide cluster {Ln 15} (Ln = Eu, Gd, Tb) with magnetic refrigerant and fluorescent properties. Polyoxometalates. doi.org/10.26599/pom.2023.9140026.