Updated by Reginald Davey 13/01/23
Ununpentium was discovered in 2003 by a group of scientists working at the Joint Institute for Nuclear Research in Dubna, Russia, and Lawrence Livermore National Laboratory (LLNL), California. The group was headed by Yuri Oganessian and Ken Moody. The term ununpentium was given on a temporary placeholder basis (the name literally means 'one-one-five').
In 2015, the International Union of Pure and Applied Chemistry (IUPAC) recognized the discovery of the element, which was renamed moscovium, referring to Moscow Oblast in Russia.
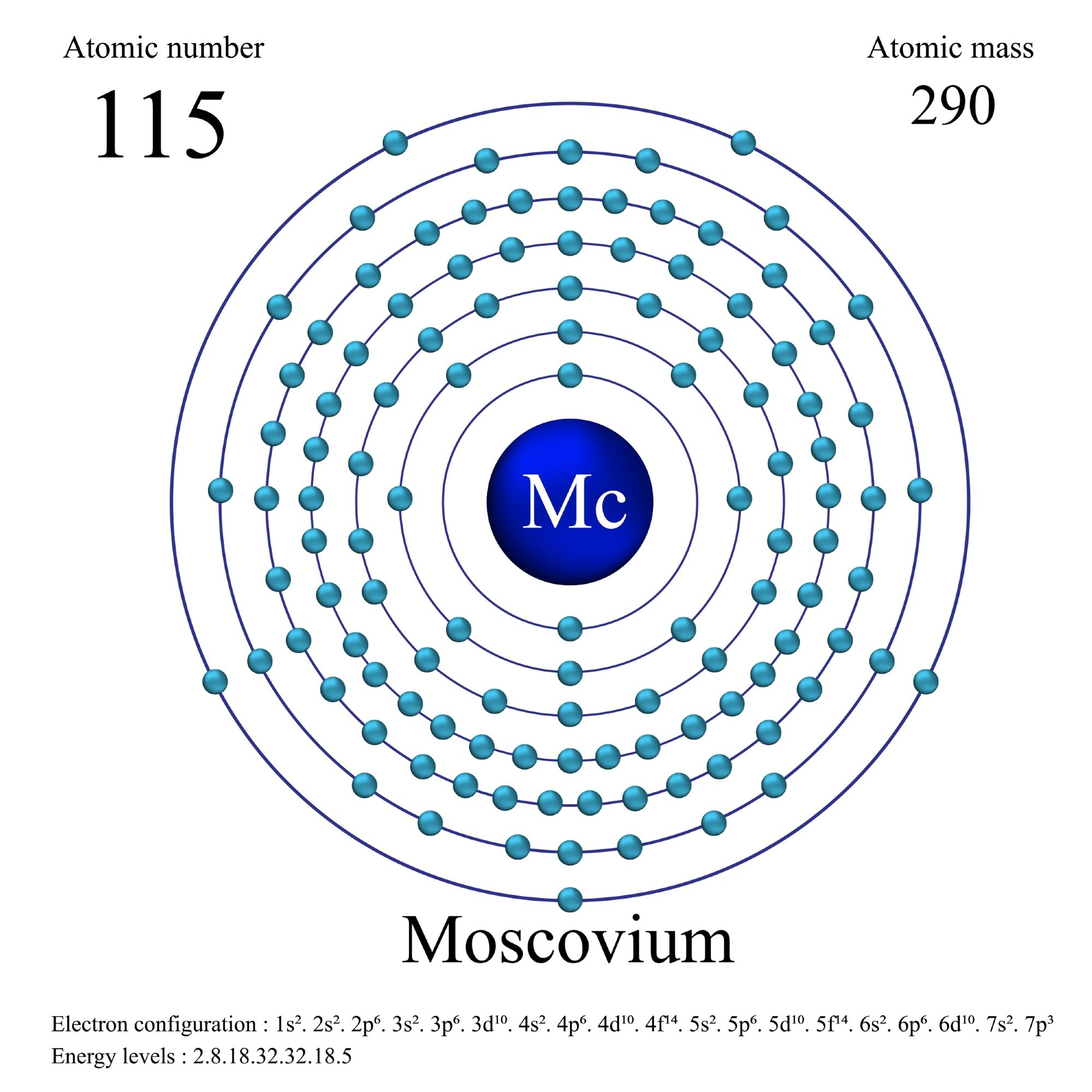
Image Credit: saran insawat/Shutterstock.com
The discovery claim was strengthened by performing experiments on the element’s final decay product, which is 268Db. Previously, the nuclides within the decay chain were unknown, so there was a lack of experimental data to support this claim.
The presence of a dubnium isotope was confirmed between June 2004 and December 2005. The scientists used chemical identification methods and measured spontaneous fission activities to confirm behavior similar to group 5 elements. The proposed 268Db’s decay mode and half-life were confirmed. Experimental results lent support to moscovium being the parent nucleus.
In the intervening years, scientists confirmed the repetition of these early experiments. Some doubt was cast upon the synthesis of moscovium in 2016, but this was addressed by the original team in 2017.
Basic Information
| Name |
Moscovium |
| Symbol |
Mc |
| Atomic number |
115 |
| Atomic weight |
[ 288 ] |
| State at room temperature |
Solid |
| CAS Registry ID |
54085-64-2 |
| Group in periodic table |
15 |
| Group name |
Pnictogen |
| Period in periodic table |
7 |
| Block in periodic table |
p-block |
| Color |
Unknown, but probably metallic and silvery white or grey in appearance |
| Classification |
Metallic |
| Melting point |
Unknown |
| Boiling point |
Unknown |
| Density |
Unknown |
Occurrence
Ununpentium (version 1) - Periodic Table of Videos
It is a highly radioactive, synthetic metallic element and only a few atoms have ever been produced. To date, around a hundred atoms of this elusive element have been observed.
Ununpentium (version 1) - Periodic Table of Videos
Isotopes
Like all synthetic elements, moscovium/ununpentium has no known naturally occurring stable isotopes. The standard atomic weight for this element cannot be given.
Moscovium has five known isotopes with mass numbers from 286 to 290. all four are unstable. However, isotope -289Mc is considered the most stable with a half-life of about 320 ms.
290Mc is the longest-lived isotope with a half-life of 650 microseconds. Isotopes undergo alpha decay into corresponding nihonium isotopes. The half-life of isotopes increases as their neutron numbers increase. 290Mc was the first moscovium isotope discovered in 2009, with 286Mc the last to be discovered in 2021.
Production
Moscovium is an artificially produced chemical element. Using equipment called a cyclotron, the scientists bombarded atoms of americium-243 with the calcium-48 ions, which resulted in one atom of ununpentium-287 and three atoms of ununpentium-288.
Health Aspects
Moscovium is considered to be harmful to health due to its radioactive nature, however, as it has very short life and is very unstable, it tends to decompose to other elements very quickly; hence it has not been possible to conduct further research to study its effects on humans.
Properties
Moscovium/ununpentium is a super heavy and artificially produced radioactive element. It is known to exist as a solid at room temperature and is the heaviest member of group 15 in the periodic table.
Currently, only the nuclear properties of muscovium have been measured. Knowledge of other properties are limited by the element’s lack of abundance and its rapid decay.
To date, key material properties such as specific heat capacity, vapor pressure, shear modulus, Young’s modulus, electron affinity, electronegativity, ionization energies are unknown.
Moscovium is expected to have similar properties to bismuth, another member of group 15 in the periodic table. In aqueous solution, moscovium’s chemistry should be that of Mc+ and Mc3+ ions.
Compounds such as moscovium carbonate and moscovium fluoride should be soluble in water. Moscovium (III) fluoride and moscovium thiozonide should be insoluble in water, much like their corresponding bismuth compounds.
Biological Role
Moscovium currently has no recognized biological role.
Applications
Moscovium/ununpentium has no applications outside scientific research. Experimental chemistry investigations have so far proven insufficient. However, pure elemental moscovium is expected to display sufficient volatility to improve the success of future chemical investigations, which may provide information on potential applications beyond the scope of research.
References
http://education.jlab.org/itselemental/ele115.html
http://www.webelements.com/ununpentium/
http://www.chemicool.com/elements/ununpentium.html
http://www.rsc.org/periodic-table/element/115/ununpentium
http://www.lenntech.com/periodic/elements/uup.htm
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.