Raman spectroscopy is a physical characterization method used widely in the pharmaceutical industry, especially in determining various polymorphic forms. The presence of water vapor, and its interaction with other materials, will influence the physical and chemical performance of medicines, excipients, and packaging materials.
Raman spectroscopy in conjunction with vapor sorption techniques enables gaining insights into vapor-solid interactions for drug materials through correlating their structural properties.
This article describes the application of the iRaman from B&W Tek, along with a Dynamic gravimetric Vapor Sorption (DVS) system from Surface Measurement Systems, to analyze the conversion of δ D-mannitol (delta form) to β D-mannitol (beta form) in real time.
Here, the in-situ monitoring of a moisture-induced polymorphic transformation is analyzed utilizing a combined Raman-vapor sorption technique. The molecular structure of D-mannitol is illustrated in Figure 1.
Figure 1. Molecular structure of D-mannitol
Experimental Procedure
Recrystallizing β D-mannitol under specific controlled conditions yielded pure α form and δ form of D-mannitol.
Mannitol is known to exist in at least three polymorphic forms, namely alpha, beta, and delta, of which the beta form has the highest stability. The less stable delta form transforms into the beta form in the presence of humidity.
The unique combination of the iRaman and DVS is shown in Figure 2.
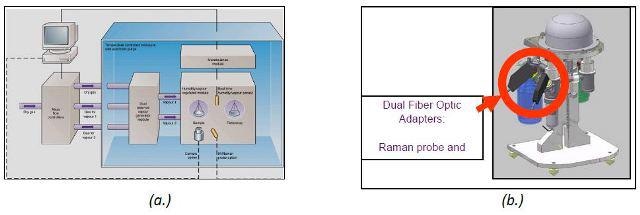
Figure 2. Schematic of dynamic vapor sorption (a.) and the DVS stand with Raman adaptor (b.).
Experimental Results
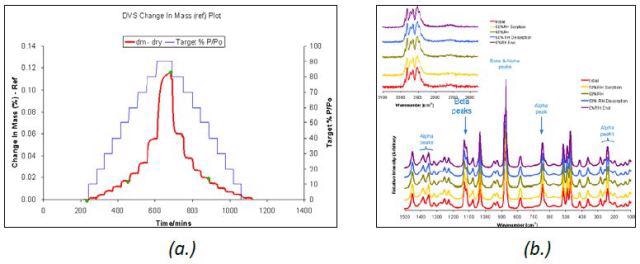
Figure 3. DVS water sorption and desorption cycle (a.) and Raman spectra (b.) for β D-mannitol.
The D-mannitol polymorph remained stable upon moisture sorption at 25°C, as illustrated in Figure 3. This was confirmed by the Raman spectra acquired during the water vapor sorption and desorption cycle.
The mixture of α form and δ form of D-mannitol remained stable upon moisture sorption. This was again confirmed by the Raman spectra acquired during the water vapor sorption and desorption cycle (Figure 4).
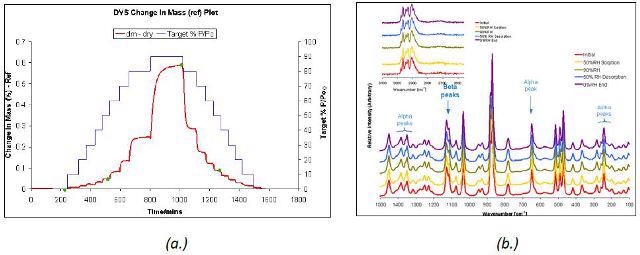
Figure 4. DVS water sorption and desorption cycle (a.) and Raman spectra (b.) for β and α D-mannitol.
A very slow change was observed in the unstable δ D-mannitol polymorph under 95% RH (Figure 5) over a period of 65 hours at 25°C, as depicted in the DVS sorption data. This was confirmed by the Raman spectra acquired during the period. This result is contrary to a complete transformation of the β polymorph within one day as observed in Figure 3.
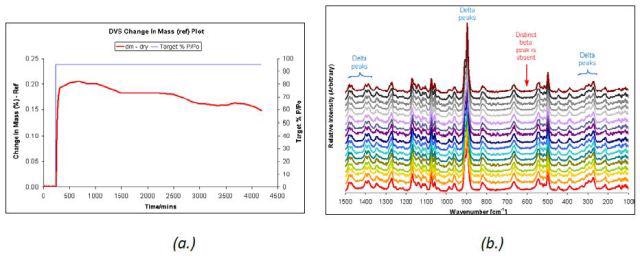
Figure 5. DVS water sorption at 95% RH (a.) and Raman spectra (b.) taken at 5-hour intervals for δ D-mannitol.
The unstable δ D-mannitol polymorph transformed rapidly under 95% P/P0 ethanol at 45°C, as demonstrated in the Raman spectra and DVS data (Figure 6). Although a complete transformation was not occurred, there was an increase in the intensity of the peaks representing the β polymorph over 24 hours.
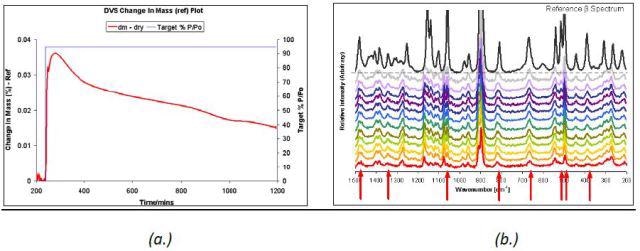
Figure 6. DVS ethanol at 95% P/P0 at 45°C (a.) and Raman spectra (b.) taken at 2-hour intervals for δ D-mannitol.
Conclusion
From the results, it is evident that Raman spectroscopy in combination with DVS is able to perform real-time monitoring of the vapor-induced polymorphic conversion of D-mannitol polymorphs.
In addition, gaining more insights into vapor-induced structural changes of pharmaceutical ingredients can be achieved using the unique combination of these two techniques.

This information has been sourced, reviewed and adapted from materials provided by B&W Tek.
For more information on this source, please visit B&W Tek.