Ensuring the reliability of the analytical technique used for generating high-quality valid data to make sound business and regulatory decisions is one of the key aspects in developing and marketing pharmaceutical drug products and dietary supplements. For this purpose, global regulatory agencies have expanded method validation specifications in recent years.
This kind of validation not only applies to traditional laboratory-based analytical methodologies such as IR, UV/Vis, GC, and HPLC, but also to portable, on-line and in-line portable devices that are deployed for rapid quality checking of products anywhere in the production process through on-board spectral libraries and intelligent, decision-making software.
Raman Spectroscopy
Raman Spectroscopy has gained traction in the pharmaceutical industry in recent years because today’s Raman equipment equipped with on-board spectral libraries and sophisticated software packages is an ideal instrument for molecular fingerprinting purposes.
Raman equipment does not require sample pre-treatment, does not involve direct sample contact, and can perform direct analysis of samples through a transparent packing material such as plastic or glass, making it suitable for use in a production environment or for remote field applications.
Over 15 years, the United States Pharmacopoeia - National Formulary (USP–NF) book of public standards has referenced Raman spectroscopy for the analysis of pharmaceutical materials. The USP has released a revision of Chapter 197 (Spectroscopic Identification Tests), which permits the application of alternative techniques like Raman spectroscopy for the in-line chemical characterization of materials, including drug compounds, excipients, or active pharmaceutical ingredients (API), in different stages of the production process.
NanoRam Handheld Spectrometer
The NanoRam from B&W Tek is an advanced, handheld Raman spectrometer designed for material identification and validation within regulatory-compliant environments. With a weight of 2.2lbs, the spectrometer enables developing standardized and validated procedures rapidly based on the established and accepted methods within the chemical industry in general and pharmaceutical, especially to facilitate purity and quality control applications.
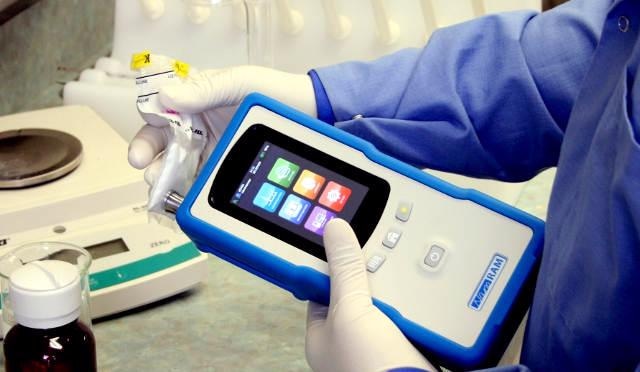
Figure 1. The B&W Tek NanoRam
After loading the device with a library of the materials to be determined and verified, the NanoRam can corroborate the identity of any material within seconds. This capability makes the device ideal for incoming raw materials verification, validation of the final product, or detecting counterfeit drugs.
Key Features of NanoRam Handheld Spectrometer
With unique synchronization capabilities, the NanoRam enables real-time wireless communication with a company’s Enterprise Resource Planning (ERP) and/or Quality Management System (QMS) software platform, thereby allowing the users to upload validation and update their devices with spectral libraries.
The unique combination of a 785nm wavelength laser excitation source, a novel temperature controlled- CCD detector, and crossed Czerny-Turner spectrometer makes the device to provide a very stable signal with low background noise and unprecedented data reproducibility.
In addition, this proprietary thermoelectric cooling CCD detector from B&W Tek in conjunction with a high-speed micro-processor and patented CleanLaze laser stabilization technology delivers laboratory-grade Raman performance in a user-friendly handheld package.
Furthermore, the NanoRam can produce a signal with a very high signal to noise specification needed for testing pharmaceutical materials.
The NanoRam provides the highest quality signal even for complex polymorphic drug compounds, thereby minimizing the requirement for repeated testing of the material. This, in turn, reduces time, improves productivity, and saves production costs.
The availability of intelligent chemometrics software packages and other software development kits (SDKs) makes the device suitable for more quantitative analyses and for more complex applications.
Regulatory Compliance
The NanoRam features B&W Tek’s proprietary NanoRam OS software, which conforms to both cGMP and 21CFR part 11certifications and satisfies all regulatory specifications for the major global testing agencies, especially related to the pharmaceutical industry, including United States Pharmacopoeia (USP), European Pharmacopoeia (EP), Japanese Pharmacopoeia (JP), Indian Pharmacopoeia (IP), and the United States Food and Drug Administration (FDA).
The NanoRam conforms to the requirements of all the major global regulatory, standards, and testing agencies with respect to the reliability, safety and calibrated operation of spectroscopic instruments employing laser technology. B&W Tek provides IQ (Installation Qualification) verification as documented proof that ensures the installation of the instrument as per the relative drawings and specifications under the guidelines of the appropriate safety regulations.
B&W Tek also offers OQ (Operational Qualification) verification to ensure the operation of all process equipment and sub-components within the specified limits and tolerances. This kind of support is offered to all equipment users, along with custom training courses on the analytical technique required for specific applications.

This information has been sourced, reviewed and adapted from materials provided by B&W Tek.
For more information on this source, please visit B&W Tek.