Sponsored by HORIBADec 16 2013
Quantum dots (QDs) have a large number of application in biolabeling, biosensing, memory devices and laser light sources. It can be seen that alloyed CdSeTe quantum dots possess a non-linear change in their photoluminescence spectra, correlated to size and composition, as monitored by the versatile benchtop FluoroMax® spectrofluorometer. The emission of quantum dots can be as long as 850nm, which may aid imaging deeper into living tissue than visible light can penetrate.
Experimental Procedure and Results
The procedure for synthesizing alloyed CdSeTe quantum dots(2.7–8.6nm dia.) is given elsewhere. Quantum dots were purified by precipitation and centrifugation, then stored at room temperature. Absorption spectra were recorded on a Shimadzu spectrophotometer (slit = 1.0nm). Fendler, et al.’s method for absorption data determined absorption onset and bandgap energies. Photoluminescence spectra were recorded on a FluoroMax®(λexc = 475nm, slits = 2.0nm bandpass). All spectra were corrected for detector response.
Quantum dots coated with tri-n-octyl phosphine oxide remain in CCl4 (lower layer) while those coated with mercaptoacetic acid are in the aqueous (upper) layer as shown in Figure 1.
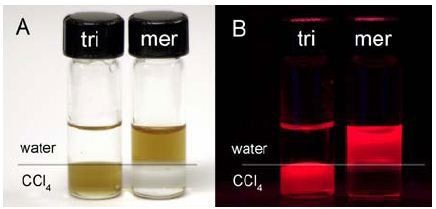
Figure 1. QDs coated with tri-n-octyl phosphine oxide (tri) and mercaptoacetic acid (mer) under ambient (A) and ultraviolet (B) illumination. The upper layer is water; the lower layer is CCl4.
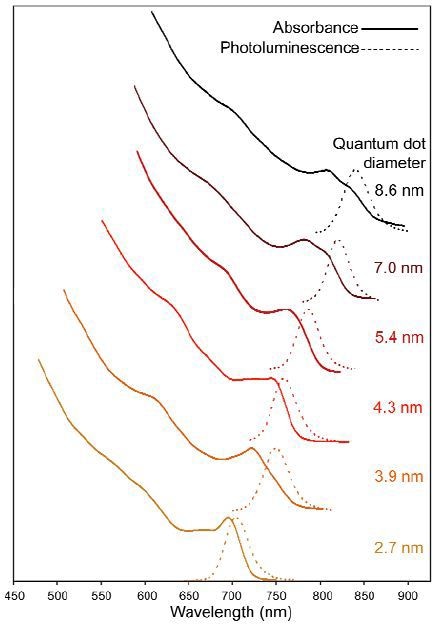
Figure 2. Diameter vs absorption and photoluminescence of various sizes of CdSe0.34Te0.66 QDs
A number of alloyed quantum dots were examined through absorption and photoluminescence spectroscopy. Absorption and photoluminescence wavelengths smoothly rise with QD diameter as shown in Figure 2. Bandgap energy is plotted against variation in Te content in Figure 3. Figure 4 shows emission peak wavelength as a function of Te content. Comparative literature values for bulk alloys are shown in Figures. 3 and 4
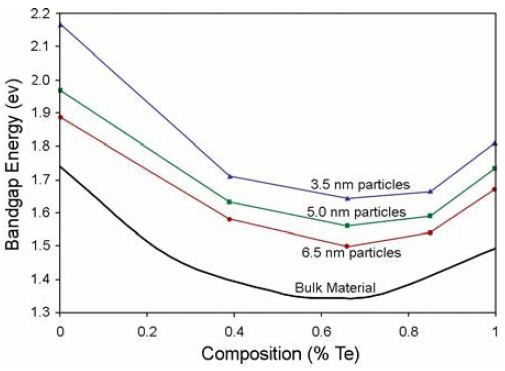
Figure 3. Absorption-energy onset vs QDs’ Te content.
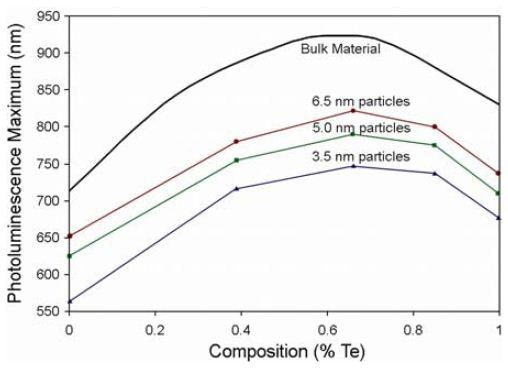
Figure 4. λem vs Te content of QDs.
Graphs resolve electronic transitions, as well as fluorescence emission at the bandedge, including an unexpected depression in band-gap for all QD sizes at ~60% Te. Vegard’s Law for predicting band-gaps of thin-film and bulk alloys is linear but only a first approximation, other have found this “optical bowing” in bulk CdSeTe, so this effect is not solely caused by quantum confinement. The observed effects arise because of various ionic sizes in the alloy, various electronegativities of these ions, and that the binary structures of these ions have various lattice constants. Relaxation of ionic bonds to equilibrium positions may lead to local order and a larger-thanexpected reduction in the band-gap.
Conclusions
Particle size and composition can control quantum confinement. Quantum dots may assist deep-tissue molecular imaging in living systems, due to their near-IR and far-red fluorescence away from aqueous absorption. QDs also provide absorption coefficients much larger thantypical organic dyes. The ultrasensitive FluoroMax® spectrofluorometer is useful for research related to nanostructures and materials science.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.