Sponsored by HORIBADec 16 2013
In organic synthesis and the manipulation of polyfunctional molecules, the choice of protecting group is very important as they can eliminate the formation of unwanted side products and reactions. Photoremovable protecting groups have a number of benefits, such as the relatively soft conditions needed for their orthogonality and deprotection with regard to base- or acid-sensitive groups. These protecting groups have found applications in synthetic organic chemistry, in the caging and releasing of compounds, and in the controlled release of a wide range of molecules in the field of materials science.
Photocleavage Reaction
In a photocleavage reaction, an ion pair from the excited ester is formed that can either split or recombine from the corresponding alcohol. A simplified scheme for an amino acid- heterocycle system is shown below.
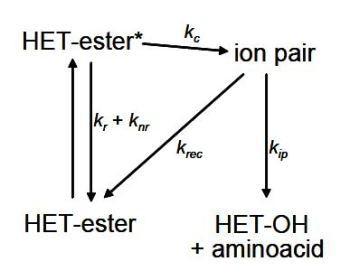
Scheme I. Simplified photocleavage scheme
It can then be the case where there will be a combination of fluorescing species, and each will potentially have a characteristic spectrum and decay time.
The aim is to achieve control over the cleavage conditions and obtain good cleavage rate, good photochemical yield and selectivity through choice of wavelength. In view of the wavelength, it is beneficial for this to be longer than ~350nm since it can prevent cell damage induced by UV light. It provides the possibility of using two-photon excitation, which is useful for biological applications, and promotes enhanced selectivity, since only a small volume will be excited.
Time-resolved Emission Spectra
It is possible that the selected system, upon photoexcitation, will include a number of spectrally overlapping species. Therefore, a simple steady state measurement will not be adequate for characterizing the system and explaining the dynamics involved. Using a time-resolved method, like the measurement of the time-resolved emission spectrum (TRES), is helpful in acquiring this data. The TRES acquisition depends on the presence of an emission monochromator. For this measurement, the HORIBA FluoroCube (Figure 1) can be used.

Figure 1. FluoroCube-01 with DeltaDiode excitation
A standard measurement involves increasing the monochromator in fixed wavelength steps, with time-resolved decays obtained for either a predetermined peak count at each wavelength or fixed time intervals. In order to achieve intensity data, the option of fixed time interval must be selected. When the instrumental response is achieved, additional analysis can be made through reconvolution. Additionally, it is possible to achieve the decay associated spectra, illustrated in Figure 2.
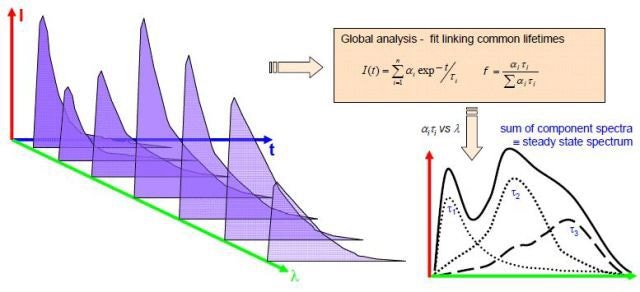
Figure 2. Scheme for obtaining decay associated spectra
Through this technique, spectra are related to the different decay times and are useful in explaining the species present.
Photocleavage of New Amino Acid Ester Derivatives
This example is based on the paper - Photolysis at long wavelengths of amino acid ester derivatives based on 4-methyl-6-methoxy-2-oxo-2H-naphtho[1,2-b]pyrans - using compounds.
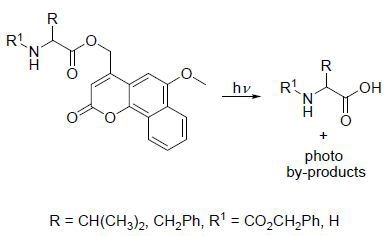
Scheme II. Compounds used and expected reaction
Time-resolved decays, which were measured with the help of a FluoroCube, demonstrated that the subsituent moieties on the amino acid have affected the decay kinetics. This is illustrated in the data shown in Figure 2. This demonstrates the influence of a substituent change on the time-resolved fluorescence behaviour. A model compound was also determined. In time-resolved fluorescence measurements, qualitative interpretation of the data can be made instantly. From the top panel, it can be observed that the decay kinetics is very different; in the other panels, DAS calculated from a TRES measurement are shown. By summing the individual DAS, the overall spectrum can be achieved. These data enable one to make comparisons and assignment of species. In the bottom panel, it is obvious that the DAS for the longer lived decay is analogous to the model compound’s spectrum.
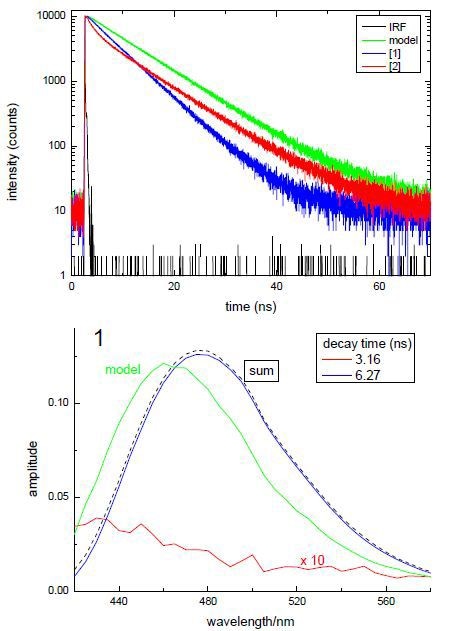
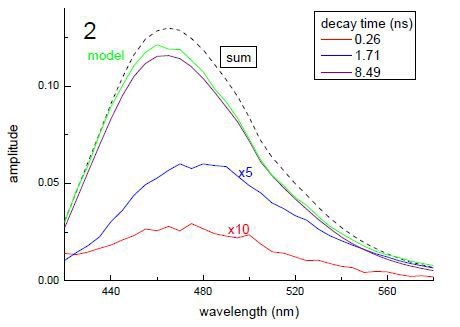
Figure 3. Example data from time-resolved lifetime and DAS analysis of compounds
Conclusion
From the lifetime values, it is possible to calculate the rate constants illustrated in Scheme I. This indicates the benefits of using time-resolved fluorescence methods in this field of application.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.