Since the discovery and structural characterization of the compound ferrocene [Fe(η-C5H5)2] in the 1950s, there was a large amount of research done on metal sandwich compound chemistry. Geoffrey Wilkinson and Ernst Otto Fischer, who defined the appropriate structure of ferrocene (Figure 1), received the Nobel Nobel Prize in Chemistry in 1973 for their work on sandwich complexes chemistry.
Ferrocene is a p-complex in which reactions between the d-orbitals of the Fe2+ metal centre with the p-orbitals of the two planar cyclopentadienyl ligands (C5H5-) form the metal-ligand bonds. Hence there is equal bonding of all the carbon atoms in the cyclopentadienyl rings to the central Fe2+ ion.
Ferrocene shows aromatic properties and is very stable. This article covers the synthesis of ferrocene and several reactions done with it as well as characterizing the products with NMR spectroscopy.
![Ferrocene [Fe(?-C5H5)2]](https://www.azom.com/images/Article_Images/ImageForArticle_11350_45037351359027782470.jpg)
Figure 1. Ferrocene [Fe(η-C5H5)2]
Preparation of Ferrocene
This experiment is done in two parts:
- Firstly freshly distilled cyclopentadiene (C5H6)is prepared
- Secondly, ferrocene is synthesized.
Both parts are done in the fumehood.
Safety
Most of the compounds, dicyclopentadiene, cyclopentadiene and 1,2-dimethoxyethane (DME) are toxic. While working with these compounds, one must take precautions to prevent contact with skin and breathe vapour.
Ferrocene Synthesis
The method of synthesis is described below:
- A 250mL 3-neck round bottom flask having a dropping funnel, magnetic stirrer and nitrogen inlet is mixed with DME (50mL) and 4.25mL of freshly distilled cyclopentadiene (Figure 2).Stir the solution and flush with nitrogen.
- Take a 1mL aliquot for 1H NMR. Add 20g finely ground KOH and stir the mixture vigorously for 15min to form a colored mixture, which includes the cyclopentadienyl anion.
- Take another 1mL aliquot for 1H NMR and prepare a finely powdered FeCl2.4H2O (5g) solution in 20mL of DMSO under nitrogen and transfer the solution into the dropping funnel.
- Add the iron(II) chloride solution slowly over a period of 30 min with efficient stirring
- Take a 1mL aliquot for 1H NMR after 2/3 of the FeCl2/ DMSO solution has been added, then another aliquot (1mL) at the end of addition.
- The reaction mixture is again stirred for 15min then the dark slurry is poured into a beaker containing crushed ice (80g) and hydrochloric acid (75mL, 6M).
- The mixture is thoroughly stirred to dissolve and neutralize any remaining KOH.
- Filter the precipitate and wash with water. Collect the crude orange ferrocene and dry in the air.
- Purify by sublimation to obtain orange crystalline material and record your yield as shown in Figure 3
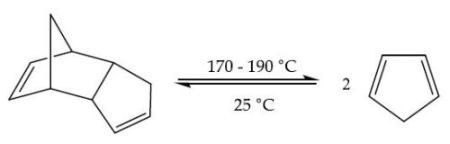
Equation 1. Cracking of dicyclopentadiene
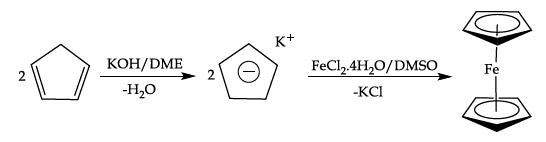
Equation 2. Formation of Ferrocene
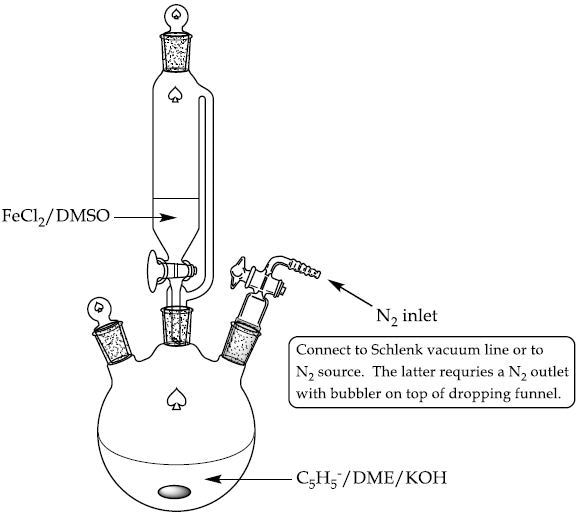
Figure 2. Experimental setup for ferrocene synthesis

Figure 3. (a)-(b). Purification of crude ferrocene via sublimation. a) Crude ferrocene (b) Sublimed ferrocene
The Spinsolve NMR spectrometer is used to monitor the different synthetic stages for preparing ferrocene. By studying the different aliquots collected during the synthetic procedure, the disappearance of reactants and formation of products can be observed. In Figure 4, the monomeric nature of cyclopentadiene (a and b), the formation of the cyclopentadienyl anion (c) and its disappearance and formation of ferrocene (d and e) are confirmed.
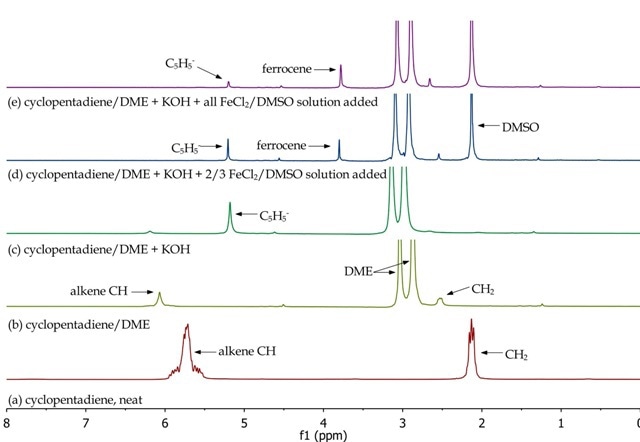
Figure 4. Overlay of 1H NMR spectra of reactants and reaction mixtures during the synthesis of ferrocene.
The 1H NMR spectrum of ferrocene (Figure 5) shows ten equivalent aromatic protons as a singlet at 4.16 ppm.
![1H NMR spectrum of ferrocene, [Fe(?-C5H5)2], CDCl3](https://www.azom.com/images/Article_Images/ImageForArticle_11350_450373513866087968786.jpg)
Figure 5. 1H NMR spectrum of ferrocene, [Fe(η-C5H5)2], CDCl3
Acetylation of ferrocene
This experiment shows the Friedel-Crafts acylation reaction to obtain acetylateferrocene.
Acetylferrocene Synthesis
The synthesis of acetyl ferrocene is as follows:
- Charge a 25mL round bottom flask with ferrocene (1g) and acetic anhydride (3.3mL).
- Add phosphoric acid (0.7mL, 85%) and heat the reaction mixture on a hot water bath for 20min with stirring.
- Pour the hot mixture onto crushed ice (27g).
- After all the ice has melted, neutralize the solution with solid sodium bicarbonate and cool for a further 5min. Collect the brown precipitate by filtration, wash with water and dry in the air.
- The solid obtained is a mixture of unreacted ferrocene and the monosubstituted product acetylferrocene (Figure 6). However, a third compound may also be present in very small amounts as the disubstituted product of acetylation (1,1'-diacetylferrocene).
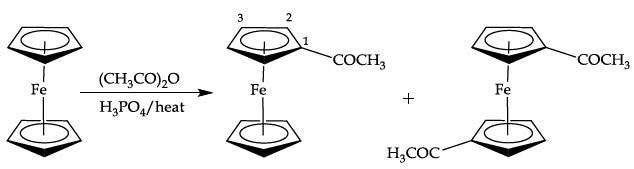
Equation 3. Preparation of acetylferrocene
Figure 6 shows the 1H NMR spectrum of acetylferrocene with five equivalent aromatic protons as a singlet at 4.19ppm for the unsubstituted cyclopentadienyl ring.
A singlet is observed at 2.39ppm (3H) corresponding to the acetyl methyl group. The substituted cyclopentadienyl ring protons are seen as a second order AA’BB’ system, with two multiplets centred at 4.49 and 4.77ppm, integrating for two protons each.
Figure 7 shows the COSY spectrum of acetylferrocene that protons at position 2 and 3 are in the same spin system, as in the substituted cyclopentadienyl ring.
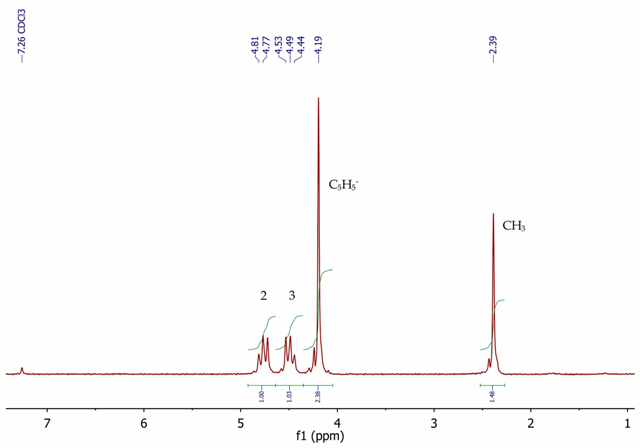
Figure 6. 1H NMR spectrum of acetylferrocene, CDCl3
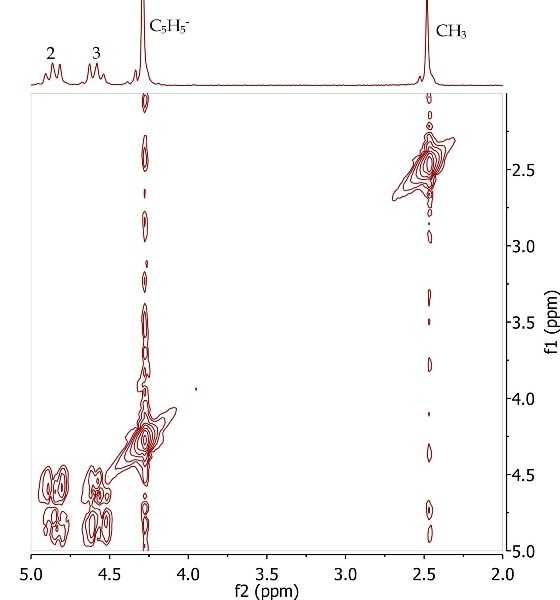
Figure 7. COSY spectrum of acetylferrocene, CDCl3. Refer to Equation 3 for the annotated structure.
Preparation of [Fe(η-C5H5)(η-C6H6)]PF6
This involves a ligand exchange reaction between one of the cyclopentadienyl rings in ferrocene and benzene to form a cationic iron p complex, which is then precipitated as the hexafluorophosphate (PF6-)salt.
Synthesis of [Fe(η-C5H5)(η-C6H6)]PF6
The synthesis method involves:
- 2.0g of ferrocene is dissolved in 10mL of benzene in a 50mL round bottom flask.
- With continuous stirring add aluminium powder (0.3g), AlCl3 (4g) and water (0.2mL).
- Place a reflux condenser atop the flask and heat the reaction mixture under reflux for 45min with efficient stirring.
- Thorough mixing of the heterogeneous reaction mixture is vital to the success of this synthesis.
- Cool the dark brown mixture in an ice bath then add ice cold water (25 mL) cautiously as considerable heat is generated.
- Place the reaction mixture in a 100mL separating funnel. The aqueous layer is collected in a beaker and made up to 50mL with water.
- Finely ground KPF6 (2.5 g) is added and the mixture is stirred for 10min.
- The green precipitate is filtered, washed with some water, ethanol and diethyl ether and allowed to dry in the air.
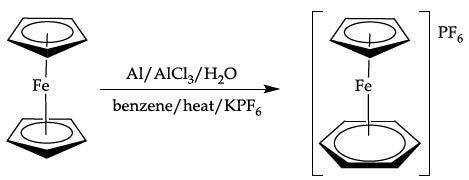
Equation 4. Ligand exchange of ferrocene with benzene
![[Fe(?-C5H5)(?-C6H6)]PF6](https://www.azom.com/images/Article_Images/ImageForArticle_11350_450373514058912042663.jpg)
Figure 8. [Fe(η-C5H5)(η-C6H6)]PF6
![1H NMR spectrum of [Fe(?-C5H5)(?-C6H6)]PF6 complex, acetone-d6.](https://www.azom.com/images/Article_Images/ImageForArticle_11350_450373514169212943331.jpg)
Figure 9. 1H NMR spectrum of [Fe(η-C5H5)(η-C6H6)]PF6 complex, acetone-d6.
Reaction of [Fe(η-C5H5)(η-C6H6)]PF6 with Nucleophiles
The reaction of the iron benzene p-complex prepared in part 3 with LiAlH4 and LiAlD4, sources of H and D- ions respectively. Arenes, for instance benzene, are more susceptible to attack by electrophiles than nucleophiles. However, associating with a metal often alters the reactivity of organic ligands. Thus, the reactivity of the benzene ligand in the iron p-complex towards the nucleophiles H- and D- is examined.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.