Malvern Panalytical, a renowned materials and biophysical characterization company, has introduced the Zetasizer Nano range of instruments that comes with the surface zeta potential cell accessory.
This accessory is available as part of a kit (ZEN1020) containing sample preparation and alignment tools, and can be utilized to measure flat surface zeta potential (Figure 1). This article enumerates the effect of temperature on silica’s surface zeta potential using the surface zeta potential cell.

Figure 1. The surface zeta potential cell kit available for the Zetasizer Nano range of instruments.
Effect of Temperature on Surface Zeta Potential
A sample holder is placed amid the electrodes of the surface zeta potential dip cell, and the surface to be determined is fixed to this sample holder. Next, the cell is submerged into a dispersant that includes a tracer particle accommodated in a square-shaped cuvette and placed within a Zetasizer Nano apparatus.
Using an adjuster placed at the top of the cell, the upright position of the sample is shifted with regard to the detection optics. When an electric field is applied, the tracer particles’ movement will be attributed to a combination of the electro-osmotic flow from the wall and the electrophoretic mobility of the particle. A simple model can be used to describe this motion and thus enable the measurement of the surface zeta potential of the specimen.
Only a few studies have been carried out relating to the association between temperature and surface zeta potential and no significant agreement was found in the published results. The following method shows how the silica’s surface zeta potential is influenced by temperature.
Methods and Materials
In this study, samples of silica were cut from a microscope slide which was uncoated, and the test plate sample was also cut to a thickness of 4mm. The space between the surface zeta potential cell electrodes was maintained at 1.5 mm in width and less than 8.0mm in length.
Solutions such as HCl, KOH and KCl were obtained from Sigma-Aldrich. For tracer particles, Coffee Compliment milk substitute in pre-dispersed form Premier Foods UK. The silica’s surface zeta potential was calculated in 1mM KCl at pH7.0 +/-0.1 pH units.
Results and Discussion
The results of surface zeta potential achieved for the silica sample as a virtue of measurement temperature is shown in Figure 2. After normalizing the surface zeta potential results to the value documented at 25°C, they are plotted against temperature. Also, the graph includes silica’s surface zeta potential values acquired from other studies.
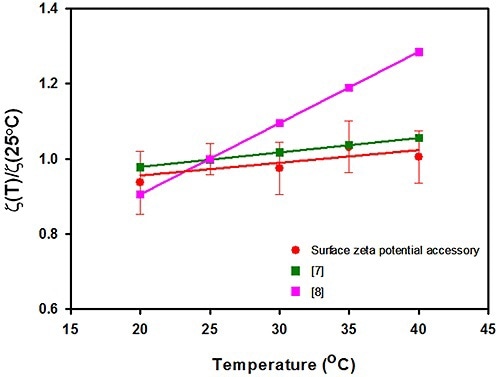
Figure 2. A plot of the surface zeta potential (in mV normalized to the value recorded at 25°C) plotted as a function of temperature (°C) for the surface zeta potential accessory used in this study. The surface zeta potential values for silica obtained from other studies.
In this study, the data predicted a slope of 0.34% variation in surface zeta potential per °C. This is in contrast to the streaming potential results which derived an association of 1.75% per °C, but is in excellent agreement with a new study performed utilizing capillary electrophoresis. The capillary electrophoresis quantified a slope of 0.39% per °C.
The error bars acquired after several measurements show that the technique can be reproduced with respect to temperature difference with a relative standard deviation (RSD) varying between 5% and 10%, and that no perceptible increase in uncertainty exists with temperature.
The entire cell, i.e. the cuvette, dispersant and surface zeta potential cell are submerged in the cell block of the instrument, and since the entire apparatus is set at the same temperature, each temperature setting is hence unimportant when compared to other methods.
Conclusion
This article briefly described the effect of temperature on silica’s surface zeta potential through the surface zeta potential cell accessory supplied with the Zetasizer Nano instrument. The results acquired from this study estimated a slope of 0.34% variation in surface zeta potential per °C.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.