Quadrupole mass spectrometers are generally used for studying the interactions of molecules with surfaces, for characterizing thin films and for demonstrating complex gas phase chemistries.
Image Credit: Shutterstock/Jens Goepfert
Both radical and neutral species determined from such processes can provide useful information regarding surface interactions and reaction pathways. Traditionally, radicals are measured by increasing the ionizer electron energy and analyzing the beginning of ionization.
This article briefly reviews the threshold ionization technique, especially the calibration technique utilized to detect unknown negative ions, which form when low electrons bind to neutral species. Figure 1 shows the threshold ionization curves for O2 and H2O, wherein O2 was initially introduced as a reference and then the commencement of ionization for the unknown gas, i.e., water vapour was evaluated against it.
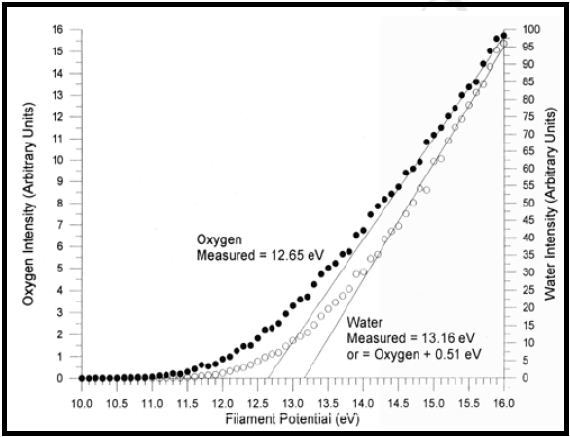
Figure 1. Threshold ionization plots for O2 and H2O.
First, the O2 data was extrapolated to the x-axis, creating a filament voltage of 12.65V. This voltage matches with the literature value of the ionization threshold of 12.06eV. Likewise, the same process for the unknown gas provided a threshold at 0.51V greater than that of oxygen. This matches with 12.57eV and is in good agreement with the literature value of 12.6eV for the ionization threshold of H2O.
In both instances, quality data is always achieved by the following parameters:
- Collection of data over a range of electron energies
- Reducing spread in electron energy
- Reducing hysteresis in the measurement
Ion Source Considerations
When ion source is utilized for low energy appearance potential (electron attachment and threshold) analysis, care must be taken to ensure that the voltage drop across the filaments is kept to a minimum. This will reduce electron energy spread and at the same time maintain the levels of emission current, as shown in Figure 2.
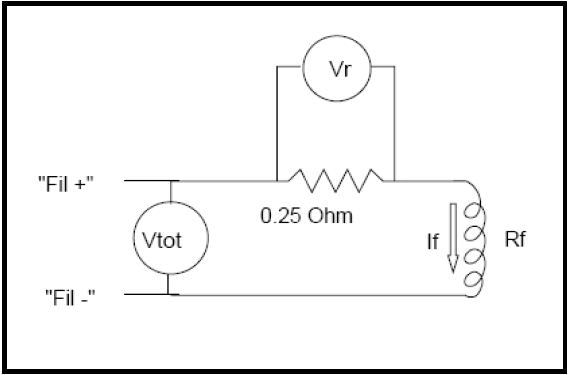
Figure 2. Filament test circuit
In the earlier work, four filaments in parallel (Figure 3) were identified, allowing a suitable compromise between emission characteristics and energy spread for work less than 10eV.
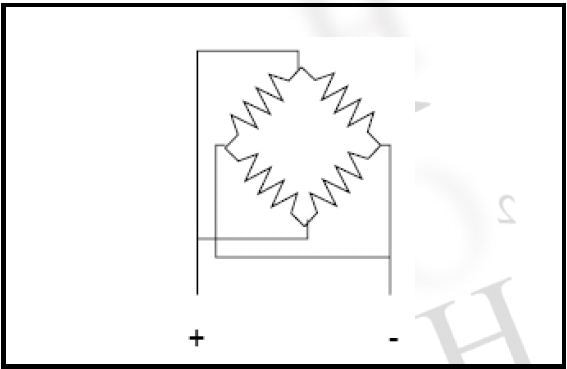
Figure 3. 4-parallel filament arrangement for threshold measurements
Attachment Processes
In materials research and processing, many electronegative gases are used such as SF6, CO2, CF4 etc. These molecules along with their fragments easily form negative ions through dissociative electron attachment, where the ensuing fragments can be different, with each process taking place at unique electron energy. This is illustrated in the example below:
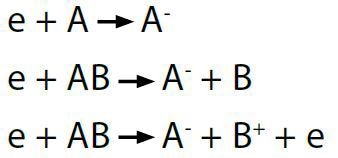
The probabilities of electron attachment for various ions have been recorded in the literature and are available through the electron attachment collision cross section data. Figure 4 demonstrates an example of the electron attachment collision cross section of CO in which O- is the negative ion detected for production at approximately 10eV.
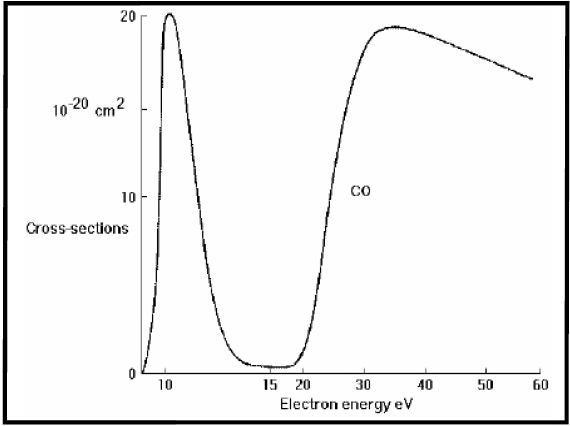
Figure 4. Electron attachment cross section for O- production from CO
CO2 Attachment Data
Figure 5 shows the electron attachment curve determined for the production of O- from CO2 and is evaluated against the literature data obtained from the plot (Figure 6). The data thus obtained is in good agreement and clearly demonstrates the capability of the mass spectrometer, in particular ion source, to differentiate between low energy electron capture events that are spaced closely.
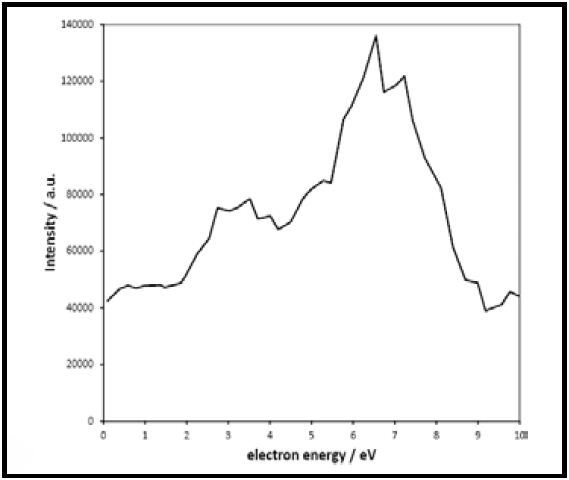
Figure 5. Measured electron attachment for production of O- from CO2
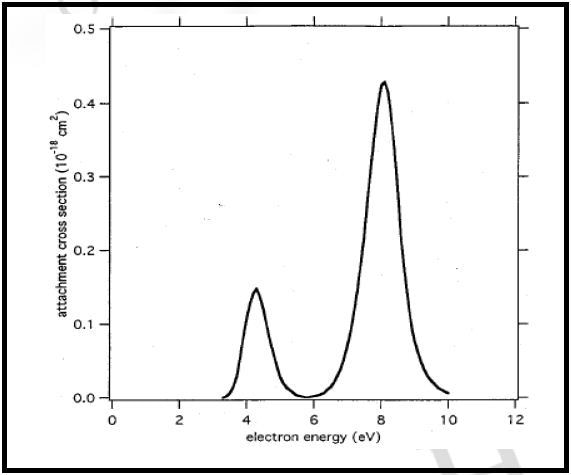
Figure 6. Literature Cross Section for production of O- from CO2
Conclusion
The Dissociative Electron Attachment (DEA-MS) plot gives data regarding the parent molecule from which the fragment ion is formed. This provides an additional level of selectivity to identify species, with significant benefits for a variety of applications.

This information has been sourced, reviewed and adapted from materials provided by Process Insights - Mass Spectrometry.
For more information on this source, please visit Process Insights – Mass Spectrometers.