Isothermal titration microcalorimetry (ITC) is a very important tool in drug discovery as well as for the regulation and study of protein interactions. Malvern Panalytical MicroCal ITC calorimeters have been specifically designed to meet the requirements of life scientists working in these sectors. The instruments offer excellent performance and superior quality data required for these applications.
MicroCal ITC systems determine the heat released or absorbed directly during a biomolecular binding event. This helps obtain a label-free direct measurement of binding affinity and thermodynamics in one experiment. Comprehensive information is offered for studying a wide range of biomolecular interactions.

Malvern Panalytical MicroCal ITC system.
Key Benefits of Malvern Panalytical MicroCal ITC systems
All MicroCal ITC isothermal titration calorimeters enable precise measurement of binding constants KD, reaction stoichiometry(n), enthalpy(ΔH) and entropy (ΔS). This offers a total thermodynamic profile of the molecular interaction.
The benefits of ITC include:
- Label-free measurement – Makes sure that analysis of unaltered biomolecules is done in their native state giving a true picture of behavior.
- Ease of use - quick to first result with minimal assay development, no labeling, no immobilization and no molecular weight limitations.
- Broad range of applications – It is possible to perform measurements under a wide variety of solvent and buffer conditions.
- Broad dynamic range – Molecule measurement in solution preserves biological relevance and the sensitivity of the technique accommodates a wide range of affinities.
- Information rich data - all relevant parameters - affinity, stoichiometry, enthalpy and entropy - are measured in a single experiment.
Malvern Panalytical offers a range of MicroCal ITC systems to suit the customer’s requirements.
- MicroCal PEAQ-ITC offers superior sensitivity and high quality data with low sample consumption. Any level of user can generate high quality data with the user-friendly guided workflows
- MicroCal PEAQ-ITC Automated combines the increased sensitivity of the MicroCal PEAQ-ITC with walkway automation in order to satisfy the productivity requirements of drug discovery and research laboratories.
- MicroCal iTC200 is developed for high sensitivity and ease-of-use. Cell injection, filling and cell cleaning functions are semi- automated and need the least amount of operator involvement.

| Model |
Sample volume |
Sample cell size |
Operation |
Throughput |
| MicroCal PEAQ-ITC Automated |
370 µL |
200 µL |
Fully automated |
Up to 42 per 24 h (SIM) |
| MicroCal PEAQ-ITC and iTC200 |
280 µL |
200 µL |
Manual |
8 - 12 per 8 h day |
Applications
The MicroCal ITC isothermal titration calorimeters are extensively used in drug discovery and life sciences with key applications in the following:
- Characterizing biomolecular interactions, in order to confirm binding and activity, measure stoichiometry and thermodynamic parameters to study structure activity relationships
- Studying the interaction of any two biomolecules including proteins, nucleic acids, lipids, drugs and inhibitors
- Drug discovery for lead optimization, Hit validation and characterization, and mechanism of action
Theory into Practice
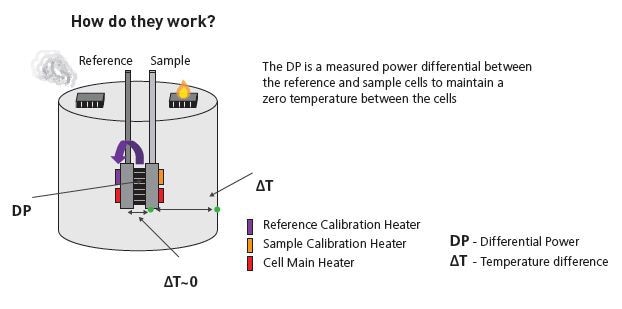
Principle of working
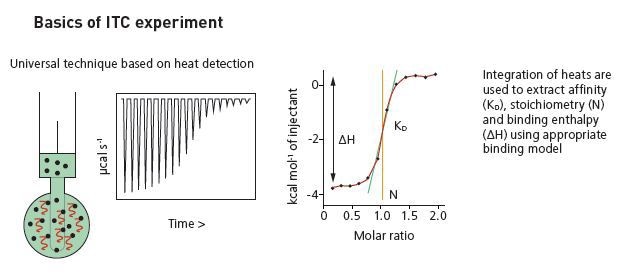
Basics of ITC experiment.
Isothermal titration micocalorimeters determine the heat change that occurs with the interaction of two molecules. Heat is absorbed or released due to the formation and redistribution of non-covalent bonds when interacting molecules move from the free to the bound state.
These heat changes are monitored by the ITC by determining the differential power, applied to the cell heaters, needed for maintaining zero temperature difference between the sample and reference cells while mixing the binding partners
The reference cell normally contains water, while the sample cell contains one of the binding partners and a stirring syringe holding the other binding partner (the ligand).
The injection of the ligand is done into the sample cell around 0.5 to 2 µL aliquots, till the concentration of the ligand is two to three times more than the sample. Each ligand injection results in a heat pulse integrated in terms of time and normalized for concentration to generate a titration curve of kcal/mol vs molar ratio (ligand/sample). The isotherm obtained is fitted to a binding model to generate the affinity (KD), stoichiometry (n) and enthalpy of interaction (ΔH).
The Power of ITC
From isothermal titration calorimetry, it is possible to obtain thermodynamic properties that tell why interactions take place. The figures A and B show how the reactions are determined.
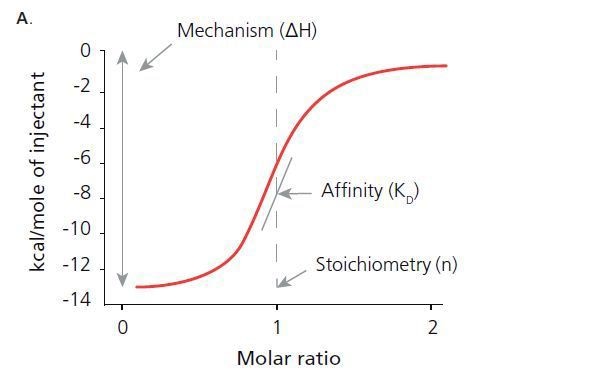
ITC determines thermodynamic properties including: the stoichiometry of the interaction (n), the afflnitycoavtantbie), change in enthalpy (ΔH), and change in entropy (ΔS).
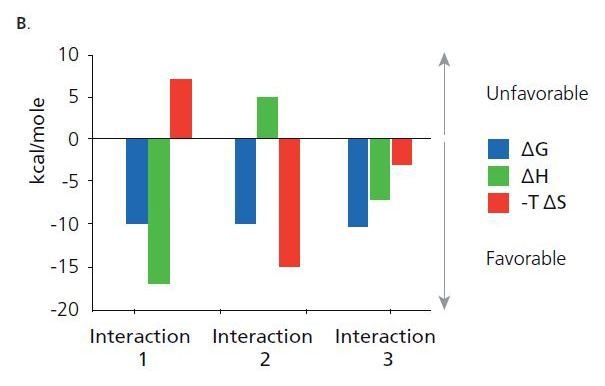
Shown are thermodynamic signatures of three interactions that have the same binding energy (AG). The binding energy is related to the affinity. Binding affinity is a combined function of the binding enthalpy (ΔH) and the binding entropy (ΔS). Binding enthalpy reflects the strength of the interaction due to hydrogen bond formation and van der Waals interactions. Binding entropy is a combination of the change in entropy from desolvation and conformational charges upon complex formation.
The advantages of MicroCal systems include:
- Minimum preparation, maximum results and high productivity
- All binding parameters (affinity, stoichiometry, enthalpy and entropy) in a single experiment
- Without any assay development results are obtained
- Coin shaped cell optimizes sample mixing
- Non-reactive Hastelloy for chemical resistance and compatibility with biological samples
- Compatible with non-aqueous solvents
- Automate for the highest productivity
- Measure sub-millimolar to picomolar diassociation constants (10-2 to 10-12 M) using direct or competitive binding techniques
- Superior sensitivity and data quality provides result confidence
- Perform a label-free, in solution investigation of any biomolecular interaction using as little as 10 µg protein
ITC in Action – Versatility Proved
An extensive number of citations in reference databases show the different applications of MicroCal ITC systems. They are used for measuring the binding affinity and thermodynamic properties of any biomolecular change that can influence recognition between binding partners. On combining with structural information, ITC data offers deep insights into structure-function relationships and the mechanisms of binding.
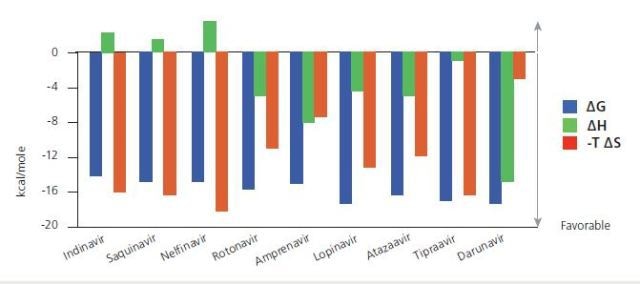
Thermodynamic signatures for a complete series of HIV-1 protease inhibitors. The signatures indicate that the most effective and recently developed drugs are more enthalpically driven than the original versions. Data from Freire, Drug Discov Today, 2008 October; 13 (19-20): 869-874.
Some applications of ITC are detailed below:
Characterizing Biomolecular Change
Using isothermal titration calorimetry it is possible to:
- Verify target activities prior to screening.
- Resolve binding into affinity, the number of binding sites, enthalpy, and entropy.
- Gain a deeper understanding of binding mechanisms for any biomolecular interaction.
Protein-Protein Interactions
These are basic to all cellular processes and when they malfunction, it is the root cause of disease. ITC was used by CHO and co-workers for understanding the role of disordered regions of the protein in molecular recognition.
This was possible by comparing a wild-type protein containing a disordered region (SEC3-WT) with three evolved variants. According to the results, it was seen that disordered regions significantly affected the binding energetics.
Optimizing Enzyme Kinetics with ITC
The kinetics of xylanase is significant in bio-bleaching wood pulp and biofuel production. Baumann and co-workers developed a system to measure xylanase kinetics with ITC since the conventional method was complex and prone to systematic errors. The ITC method was also found to offer higher sensitivity and used less material.
Typically, heat changes associated with xylan hydrolysis are too small to determine directly with ITC. In order to increase heat flow, an enthalpy amplification system involving carbohydrate oxidase and catalase was developed.
This generated an enthalpy change that enabled measurement by MicroCal iTC200, with signals that corresponded to the amount of mixed xylan oligosaccharides injected.
Zinc-Induced Dimerization of a Chaperone
ITC was used by Zambeli and coworkers to understand the role played by disordered regions of UreG, from Helicobacter. UreG is a molecular chaperone, which activates urease by providing two nickel ions.
The procedure employed GTP hydrolysis and used a number of metal-ion interactions and several proteins. As UreG is not structured in solution it has been tough to understand the structure function relationships.
The stoichiometry showed that two nickel ions linked to each protein in process does not need protein dimerization.
Predictive Power and Productivity in Fragment-Based Drug Discovery
An automated MicroCal ITC was used for optimizing and determining in an potential lead compounds in an early drug discovery program for treating drug resistant tumors. Data obtained from an automated MicroCal ITC was used to confirm hits from a primary screen and to precisely rank the affinities of the fragments in such a way that only the strongest binders were chosen for co-crystallization attempts and structure-based drug discovery program.
It was successfully forecast from this approach which hits would form co-crystal complexes with the target. This was shown clearly when 12 of the 14 protein complexes selected from the ITC validation were crystallized successfully.This study shows the predictive power of ITC can streamline a +- fragment based drug discovery (FBDD) workflow and save time.
MicroCal ITC Range
MicroCal PEAQ-ITC Automated
This instrument integrates the superior performance of the MicroCal PEAQ-ITC with full automation and operation without user intervention, the MicroCal PEAQ-ITC Automated is a valuable asset for any busy research laboratory.
User-friendly software makes sure that efficient experimental design is achieved while automated data analysis enables rapid and reliable results. The throughput and automation offers makes it an excellent choice for drug discovery applications such as hit validation in cases where productivity is critical.
Key Features
- Completely automated with capacity to run four 96-well plates without user intervention
- Optimized automation scripts for better performance and assay reliability
- Single syringe load for multiple titrations (i.e. 4 experiments of 10 µL)
- New simplified layout
- Software which streamlines workflows and improves data analysis consistence for confident decision- making
- Append new experiments 'on the run' to increase productivity
MicroCal PEAQ-ITC
MicroCal PEAQ-ITC is developed for high sensitivity and convenience. The broad affinity range allows analysis of weak to high affinity binders with very good repeatability.
MicroCal PEAQ-ITC analysis software enables experiment design simulation, automated assessment of data quality, batch evaluation of large data sets and a streamlined user interface that enables the user to finalize figures and presentation quality graphs conveniently.
MicroCal ITC is an important tool for any research laboratory studying biomolecular interactions where rapid results and high sensitivity are very essential.
Key Features
- User-friendly guided workflows having embedded video tutorials offering any level of user the ability to generate high quality data
- High signal to noise provides more confidence in accessing data quality and relevance of generated affinity and thermodynamic parameters
- Automated washing with sample cell detergent and titration syringe helps produce high quality reproducible data
- Studies all binding parameters (affinity, stoichiometry, enthalpy, entropy) in a single experiment
- Quick to first result with the least assay development and no labeling
- Sensitive enough to investigate biomolecular interaction using as little as 10µg protein
- Directly measures millimolar to nanomolar affinities (KDS) (10-2 to 10-9 M)
- Measures nanomolar to picomolar disassociation constants using competitive binding techniques (10-9 to 10-12 M)
MicroCal PEAQ-ITC Analysis Software
The key features of the software include:
- Open multiple experiments in a single session
- Automated fitting models (One-Site, Two-Site, Sequential, Competitive, Enzyme Kinetics, Dissociation)
- Automated assessment of data quality
- Good quality data - Binding
- Good quality data - No binding
- Poor quality data - Check data
MicroCal iTC200
The MicroCal iTC200 is developed for convenience and very high sensitivity. Its broad affinity range helps analysis of weak to high affinity binders with a very good repeatability. Syringe and cell cleaning functions are semi- automated and need the least operator involvement.
User-friendly software guides all operations for fast accurate analysis. MicroCal iTC200 is a very important tool for any research laboratory studying biomolecular interactions where high sensitivity and rapid results are highly significant.
Key Features
- All binding parameters (affinity, stoichiometry, enthalpy, entropy) in a single experiment are analyzed
- Quick to first result with very less assay development and no labeling
- Sensitive enough to investigate biomolecular interaction using as little as 10µg protein
- Directly measures millimolar to nanomolar affinities (KDS) (10-2 to 10- 9 M)
- Measures nanomolar to picomolar disassociation constants using competitive binding techniques (10-9 to 10-12 M)
Step-by-Step Experiment
The step-by-step experiment is shown in the figures below:





Specification Comparison Summary
| Parameter |
PEAQ-ITC Automated |
PEAQ-ITC |
iTC200 |
| Measurement parameter |
Affinity (KD) |
Affinity (KD) |
Affinity (KD) |
| Measurement parameter |
Enthalpy ΔH |
Enthalpy ΔH |
Enthalpy ΔH |
| Measurement parameter |
Entropy ΔS |
Entropy ΔS |
Entropy ΔS |
| Measurement parameter |
Stoichiometry (n) |
Stoichiometry (n) |
Stoichiometry (n) |
| Sample capacity |
384 ( four 96 well plates) |
N/A |
N/A |
| Sample tray temp range |
4°C± 2°C at ambient |
N/A |
N/A |
| Sample volume |
370 pL |
280 pL |
280 pL |
| Cell volume |
200 pL |
200 pL |
200 pL |
| Injection syringe volume |
40 pL |
40 pL |
40 pL |
| Injection volume precision |
< 1% @ 2 pL |
< 1% @ 2 pL |
< 1% @ 2 pL |
| Equilibration time from 25 °C to 5 °C |
< 6 min |
< 6 min |
< 6 min |
| Sample presentation |
96 well plate |
N/A |
N/A |
| Throughput |
Up to 42 per 24 h (SIM) |
8-12 per 8 h day |
8-12 per 8 h day |
| Cell material |
Hastelloy |
Hastelloy |
Hastelloy |
| Cell configuration |
Coin-shaped |
Coin-shaped |
Coin-shaped |
| Noise |
0.15 ncal/s |
0.15 ncal/s |
0.2 ncal/s |
| Temperature Range |
2°C to 80°C |
2°C to 80°C |
2°C to 80°C |
| Temperature stability at 25 °C |
± 0.00012°C |
± 0.00012°C |
± 0.00015°C |
| Response time |
10 s |
10 s |
10 s |
| Multiple feedback modes |
Yes (passive, high gain, low gain) |
Yes (passive, high gain, low gain) |
Yes (passive, high gain, low gain) |
| Automated upgrade available |
N/A |
Yes |
Yes |
| Operating Environment |
| - Temperature range |
10°C to 28°C |
10°C to 28°C |
10°C to 28°C |
| - Humidity |
0% to 70% RH, non condensing |
0% to 70% RH, non condensing |
0% to 70% RH, non condensing |
| Electrical ratings |
| - Voltage |
100 - 240V |
100 - 240V |
100 - 240V |
| - Frequency |
50/60 Hz |
50/60 Hz |
50/60 Hz |
| - Power |
70 W |
70 W |
70 W |
| Weight |
91 kg |
13.6 kg |
9.4 kg |
| Dimensions (W x H x D) |
63 x 77 x 35 cm |
43 x 46 x 38 cm (calorimeter + wash station) |
21 x 34 x 35 cm (calorimeter) |

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.