Sponsored by HORIBAJun 14 2018
Oxygen (O2) dissolved in water is called dissolved oxygen (DO). In the natural world, the amount of O2 dissolved in water is proportional to the partial pressure of O2 in air and is expressed as the amount of O2 dissolved per unit volume of water (mg/L). The saturation level of O2 dissolved in pure water at 25 °C and 1 atm is 8.11 mg/L (JISK0102-2010) and decreases with an increase in temperature.
What is Dissolved Oxygen?
Fish and other aquatic organisms survive in water because of DO. The amount of DO in water is affected by the water temperature, salt concentration, and atmospheric pressure, among other factors.

Where water is polluted with high levels of organic pollutants and a high BOD or COD1, there is a decrease in the amount of DO because a large amount is consumed by aerobic microorganisms that decompose the organic matter. Hence, in rivers that are greatly polluted with organic matter, necrosis of aquatic life tends to occur in hot summers.
HORIBA’s Multiparameter Water Quality Checker
Between May 28, 2009 and June 9, 2009, a Multiparameter Water Quality Checker (model:U-50) from HORIBA was installed near the surface of Inbanuma (Inba Marsh), a marsh in Chiba prefecture, to continuously measure DO levels.2 Figure 1 shows the DO and turbidity measurements and Figure 2 shows the pH measurements and ORPs. DO levels increased in the morning and during the day and decreased in the evening and night. This pattern was observed consistently during the period of measurement.
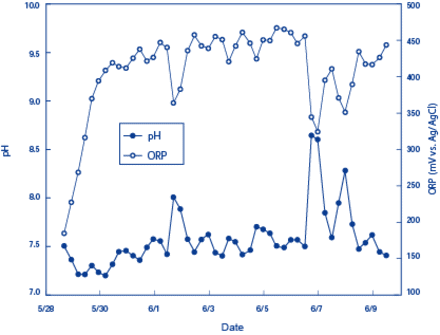
Figure 1. Measurement Results of pH and ORPs in Inba Marsh (May 28 to June 9, 2009)
On June 1, 2, 4, and 6, when peak levels of DO were detected (Figure 1), there were no or very few clouds in the sky. This may reflect the oxygen production by photosynthesis by aquatic plants during the day and their oxygen consumption by respiration during the night.
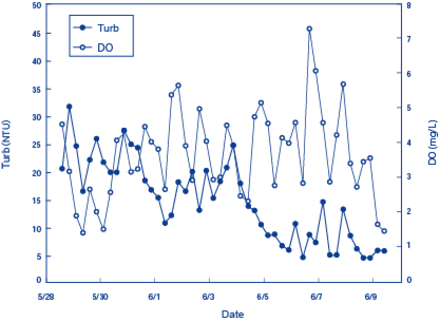
Figure 2. Measurement Results of DO and Turbidity in Inba Marsh (May 28 to June 9, 2009)
References and Further Reading
- Biochemical oxygen demand (BOD) is the amount of oxygen required to decompose organic pollutants in water, and chemical oxygen demand (COD) is the amount of an oxidant required to decompose the organic pollutants. Both are indicators of organic matter pollution of water.
- “Development of the U-50 Series Multiparameter Water Quality Checker,” Yuichiro Komatsu, Katsunobu Ehara, and Katsuaki Ogura, HORIBA Technical Report, Readout, No. 35, and 56–61 (2009).

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.