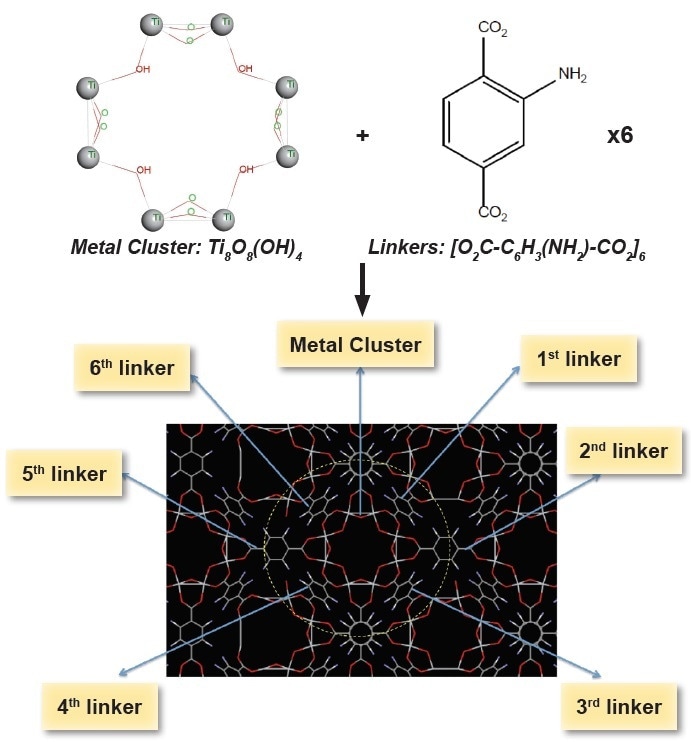
NH2-MIL-125(Ti), AYRSORB™ T125 the metal-organic framework (MOF) material—consists of a repeating unit cell. This unit cell consists of six linkers (ligands) and a metal cluster. The linkers form a porous network by joining the metal clusters together which is known as a MOF. To clarify the structure in Figure 1, the cluster is illustrated with its elemental components to help the analyst match the structure to its formula as shown below.
- CAS# 1309760-94-8
- Formula: C48H34N6O36Ti8
- Formula Weight: 1653.74
- Color and Form: Yellow powder
- Available Sizes: 250 mg, 1 g
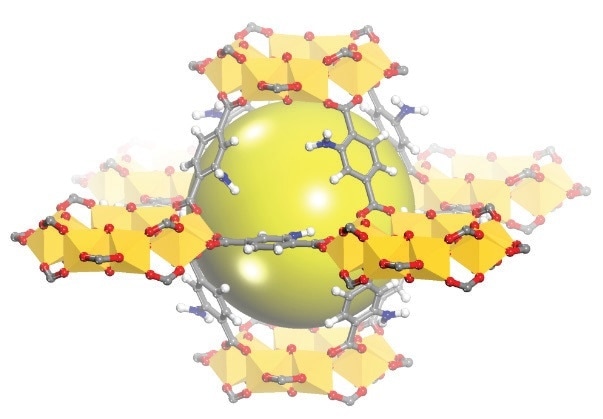
Figure 1. Schematic view of the cages of MIL-125(Ti)-NH2
Isolation of Solid Denoted MIL-125(Ti)-NH2
Using a suitable choice of solvent mixtures (methanol and dimethylformamide (DMF), solid denoted Ti8O8(OH)4(2OC-NH2-C6H3-CO2)6·18(CH3OH)·3((CH3)2NCHO) or MIL-125(Ti)-NH2 has been separated (MIL is the acronym for Material from Institute Lavoisier). The synthesized solid was heated under vacuum at 200°C for 6 hours to remove the solvent molecules (see Thermogravimetric analysis in Figure 2).
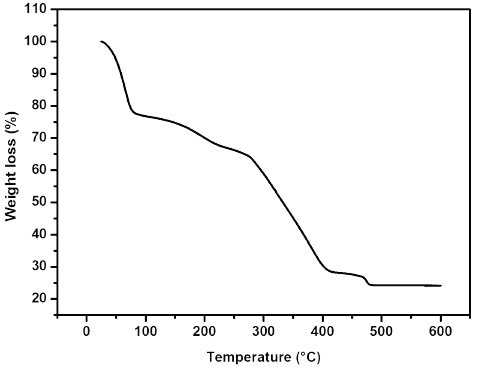
Figure 2. Thermogravimetric analysis
MIL-125-NH2 is thermally powerful. X-ray diffractometry (Figure 3) does not signify any variation in crystallinity after the departure of the guest molecules at less than 200 °C.
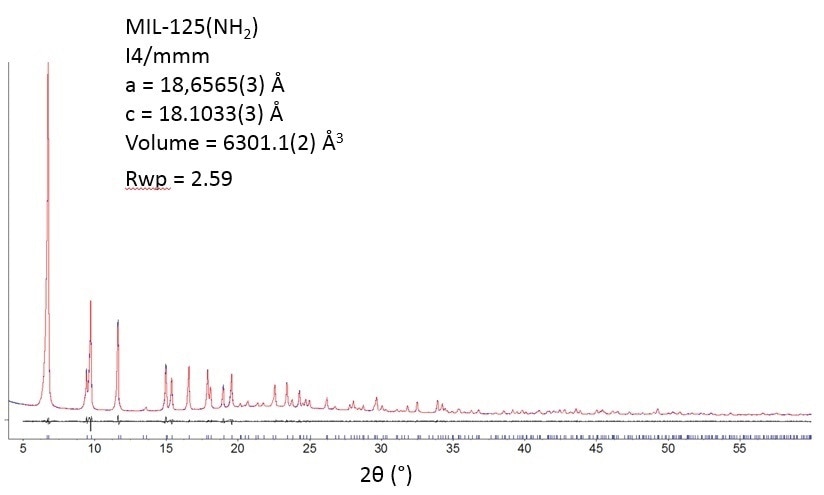
Figure 3.
Nitrogen sorption experiments show that MIL-125-NH2 is a highly porous property of microporous solids, a micropore volume (Vp) of 0.74(2) cm3·g-1, and a BET surface area of 1530 m2·g-1 (Figure 4).
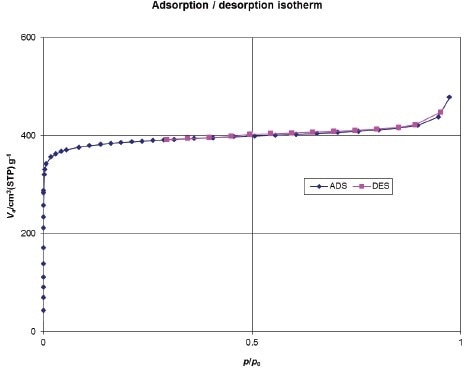
Figure 4.
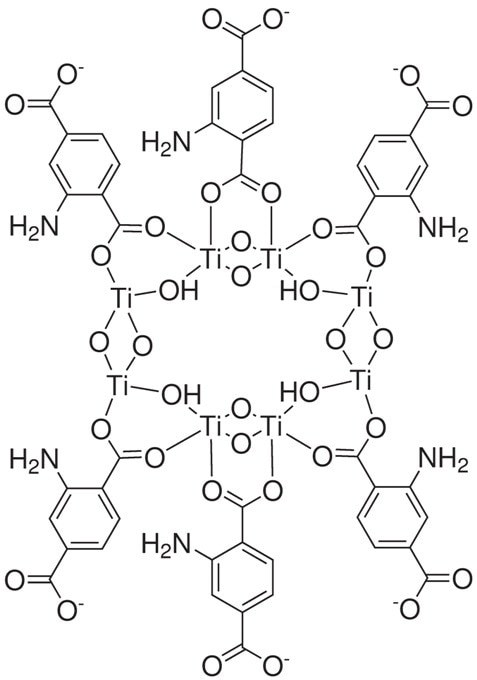
Figure 5. Structure drawing of 22-1070
Activation Protocol
This material can be activated by heating at 200 °C for 6 hours under vacuum. Upon cooling under vacuum, the activated product must be stored and handled in an inert atmosphere.
References and Further Reading
- A New Photoactive Crystalline Highly Porous Titanium(IV) Dicarboxylate, Meenakshi Dan-Hardi, Christian Serre, Theo Frot, Laurence Rozes, Guillaume Maurin, Clement Sanchez, and Gerard Ferey, J. Am. Chem. Soc., 2009, 131, 10857–10859.
- A robust amino-functionalized Titanium (IV) based MOF for an improved separation of acid gases, Sébastien Vaesen, Vincent Guillerm, Qingyuan Yang, Andrew Wiersum, Bartosz Marszalek, Barbara Gil, Alexandre Vimont, Marco Daturi, Thomas Devic, Philip L. Llewellyn, Christian Serre, Guillaume Maurin and Guy De Weireld, Chem. Commun., 2013, 49, 10082-10084.
- Adsorption/catalytic properties of MIL-125 and NH2-MIL-125, Se-Na Kim, Jun Kim, Hee-Young Kim, Hye-Young Cho, Wha-Seung Ahn, Catalysis Today 2013, 204, 85– 93.

This information has been sourced, reviewed and adapted from materials provided by Strem Chemicals.
For more information on this source, please visit Strem Chemicals.