Hydrogen is understood by many to be the superior carrier gas for gas chromatography (GC), and in some applications, its individual properties - rapid analysis, optimal efficiency and low costs - make it the gas of choice. Nevertheless, helium remains the most popular choice. This may change, however, as temporary helium supply restrictions cause chromatographers to reconsider hydrogen and its benefits to GC.
Helium remains a solid choice for a carrier gas in GC however, although there are sufficient reserves for several hundreds of years, the utilisation of hydrogen is growing and GC manufacturers are developing new instruments optimised for hydrogen carrier gas. In contrast to helium, hydrogen is flammable, but its high diffusivity permits more rapid linear velocities and shorter analyses, while still offering a similar separation efficiency as helium.
To explore the effect of carrier gas on resolution and analysis time, a Supelco isothermal non-polar test mixture was tested in helium, hydrogen and nitrogen. Each carrier gas was calibrated at its optimal linear velocity at the starting temperature of the temperature ramp. There are observable differences in the retention times and resolution. The column and conditions were unchanged. Lower duration analyses allowed increased throughput and reduced costs per sample.
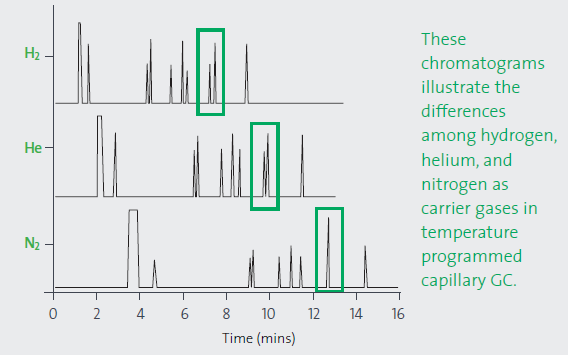
Figure 1. Effect of carrier gas on resolution and analysis time
Nonetheless, when using hydrogen as the mobile phase, there are potential concerns relating to reactivity in certain situations (catalytic hydrogenation of unsaturated molecules at high inlet temperature). This potential for chemical reactions in your analytical system must be considered.
The Air Products Solution – Hydrogen BIP®
To satisfy the demand for hydrogen as a carrier gas, Air Products has now extended its unique BIP® technology to hydrogen. Hydrogen BIP® cylinders are available with ultra-low impurity specifications, not previously available from cylinder or generator sources. (See table below)
| Grade |
H2O |
O2 |
THC |
Certification |
| H2 BIP® cylinder |
20 ppb |
100 ppb |
10 ppb |
Batch |
| H2 BIP® PLUS cylinder |
20 ppb |
100 ppb |
10 ppb |
Individual |
THC = Total Hydrocarbons measured as Methane
The unique BIP® cylinders manufactured by Air Products use an advanced technique to remove critical impurities as the gas is withdrawn from the cylinder. This yields a gas with superior purity levels, which is suitable for the most exacting gas chromatography functions. Every H2 BIP® cylinder contains ultralow levels of impurities, with not more than 20 ppb of moisture; 100 ppb of oxygen and 10 ppb of total hydrocarbons. This ensures that hydrogen BIP® gas is one of the purest grades available.
Making the Helium to Hydrogen Switch
When changing from helium to hydrogen, the adjustments required by the gas chromatograph correspond to the hazards of hydrogen. Hydrogen is flammable and can generate an explosive atmosphere when it gathers. The priority is to maintain safety standards and ensure there is no accretion of gas by preventing and detecting leaks, and safely venting any hydrogen outlet stream.

Avoiding Gas Build-Up
Contemporary gas chromatographs can identify leaks upstream in the column by observing gas pressure. This can be implemented directly for a GC operating with hydrogen. If hydrogen leaks prior to reaching the column, the GC pressure drop is minimal; hydrogen pressure cannot build up and it does not arrive at the defined set point. The GC interprets the permanent divergence between the operative and the set point pressure as a leak and safeguards the GC by restricting the hydrogen valve. Conversely, when the leak is downstream in the column, this protection is inefficient and a hydrogen sensor would need to be integrated to identify any hydrogen build-up in the oven.
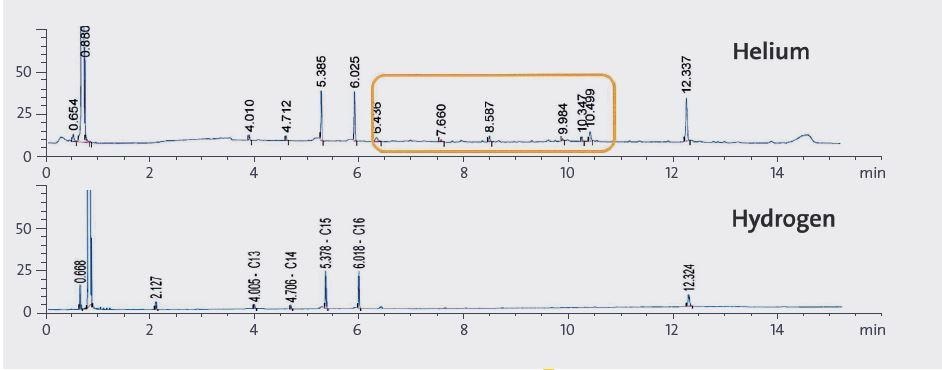
Figure 2. 0.5 uL sample chromatograms using helium or hydrogen as carrier gas
Safe Venting
The second adjustment required when switching from helium to hydrogen is to safely vent the outlet stream. The relevant ports are the outlet of the septum purge and the split from the injector. This is important because when helium is utilised it can be vented from within the laboratory, but hydrogen requires the ports to carry it to the laboratory’s flammable vent line.

Introduction of a flow restrictor upstream of the pressure regulator, restricts the maximum leak possible and guarantees that laboratory ventilation can dilute hydrogen leaks. Flow restrictors are a low cost, functional and efficient way of limiting this type of gas leakage. Once these measures are tackled, the new GC configuration can be authenticated by implementing a standard FID performance evaluation test.
Hydrogen Cylinders or Generators?
Hydrogen generators might present an alternative for GC carrier and fuel gases, and their relative advantages and disadvantages should be compared alongside cylinders before deciding on a preference. H2 generators have two principal advantages:
- They are a solid solution in remote locations when cylinder supply is restricted or non-existent.
- They generate H2 on demand, so little H2 is stockpiled.
However, H2 generators are not always the best solution, and there are often hidden disadvantages:
- Gas Specification – This should be scrutinized meticulously, as most manufacturers only indicate either the O2 or H2O impurity levels, but not both.
- Cost - H2 generators are typically more expensive than an H2 cylinder supply.
- Reliability and Back-up - H2 generators can fail disastrously without warning, rendering back-up cylinder supply as vital.
- Specialist Equipment - Deionizer bags purify the demineralised water supply. These must be changed regularly or the generator will be seriously damaged.
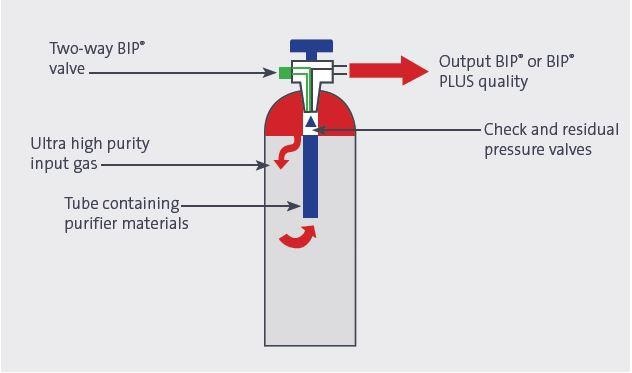
Figure 4. BIP® valve and purifier design
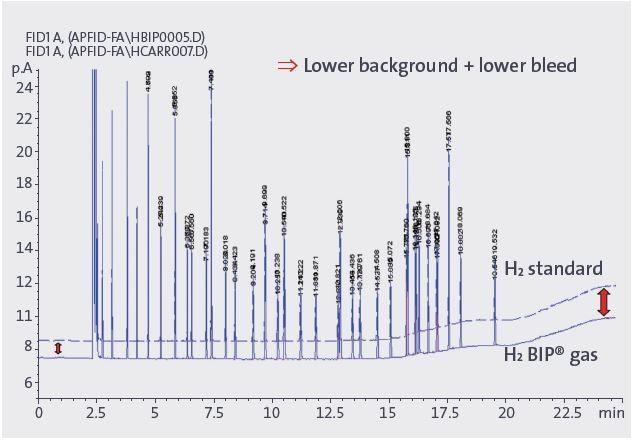
Figure 5. Baseline noise reduction and column bleed improvement with simultaneous fuel and carrier gas change from H2 standard to H2 BIP® gas
Safety Systems
An undetected gas leak can materialise within a broken column or a leaking connection regardless of whether a cylinder or generator is providing the carrier gas. The danger is that an undetected gas leak could generate an explosion in the GC oven, placing laboratories and operatives at risk. It is thus critical, for any laboratory utilising hydrogen as a carrier gas, to be able to safely identify hydrogen leaks in the GC oven.
H2 sensors, which ensure the safe use of hydrogen in GC analysis, are available from all leading GC vendors. The H2 sensor performs its function by continually examining the H2 concentrations in the GC oven, and automatically shifting to an inert gas when, typically, 25% LEL is attained. This important feature reduces hazards and maintains safety.

This information has been sourced, reviewed and adapted from materials provided by Air Products.
For more information on this source, please visit Air Products.