Using Gas Chromatography for the Analysis of Ultra Low Sulfur Compounds in Gaseous Fuels
- Robust solution utilizing AC SeNse detector
- Ultra-low detection limits
- Superior sensitivity, linearity, and repeatability
Introduction
This article outlines the identification of speciated volatile sulfur-containing compounds in high methane content gaseous fuels such as natural gas, utilizing the innovative PAC SeNse detector.
The SeNse detector has a high sensitivity to sulfur response, equimolar, linear, and no quenching or interference from co-eluting hydrocarbons is seen. Several sources of petroleum and natural gases include sulfur compounds, which are corrosive, poisonous, and odorous to catalysts utilized in the processing of gaseous fuel.
For the purpose of safety, low ppm quantities of sulfur odorants are introduced to LP gases and natural gas. Certain odorants are unstable and react to produce compounds with smaller odor thresholds. Quantitative investigation of these odorized gases verifies that odorant injection instruments perform to standard.
Fuel gases are additionally employed in energy production or are changed into novel products utilizing catalysts that have been poisoned by an excess of sulfur in the feed gas. Industries often require the quantification of sulfur in these fuel type gases to protect their investments in catalysts.
Instrumental
The sample loop is switched in the carrier gas stream to add the sample onto the analytical column. The thick phase methyl silicone capillary column divides the trace sulfur components from each other and from the matrix in a temperature-controlled run.
The capillary column is integrated with a dual plasma furnace where the sulfur compounds are combusted to SO2. In the presence of excessive hydrogen, sulfur dioxide is reduced to a range of reduced sulfur species.
The reduced sulfur species are moved to a reaction cell. The reaction cell is filled with ozone, which reacts with the reduced sulfur species to produce exited state sulfur dioxide. A photon is released upon the relaxation of sulfur dioxide to the ground state. A photomultiplier tube is employed to quantify the light emitted which is then converted to a voltage.

Figure 1. Plumbing diagram for Sulfur Compounds in Natural Gas and Gaseous fuels analyzer according ASTM D5504 using PAC SeNse detector.
Validation
The methodology and system of the AC ASTM D5504 Ultra low PAC SeNse detector are rigorously investigated for repeatability, separation efficiency, detection levels, equimolarity, recovery, and response linearity.
Separation Efficiency
Chromatographic surroundings are enhanced to acquire effective separation of the common Sulfur compounds. As demonstrated in Figure 2, the column achieves baseline separation for carbonyl sulfide and hydrogen sulfide at an initial oven temperature of 35 °C (with no cryogenic cooling necessary).

Figure 2. Calibration gas (~10 ppm single compound).
Repeatability
Retention time and area (concentration) are the two main measurements in gas chromatography. The accuracy in which they are quantified ultimately confirms the credibility of the quantitative data produced.
Area and retention time precision is reliant on all parameters (pressure, flow, injection, temperatures) being managed to exacting tolerances. The area precision can be significantly affected by the inertness of the flow path, particularly for active sulfur components at low levels.
Retention Time Repeatability
ASTM D5504 explains: “Chromatographic parameters must be capable of obtaining retention time repeatability of 0,05 min (3s) throughout the scope of this analysis.”
The repeatability of retention time is quantified for 8 runs consecutively for a calibration standard blend diluted to 500 ppb single peak (Figure 3). Table 1 demonstrates the calculated retention time repeatability of the sulfur components.
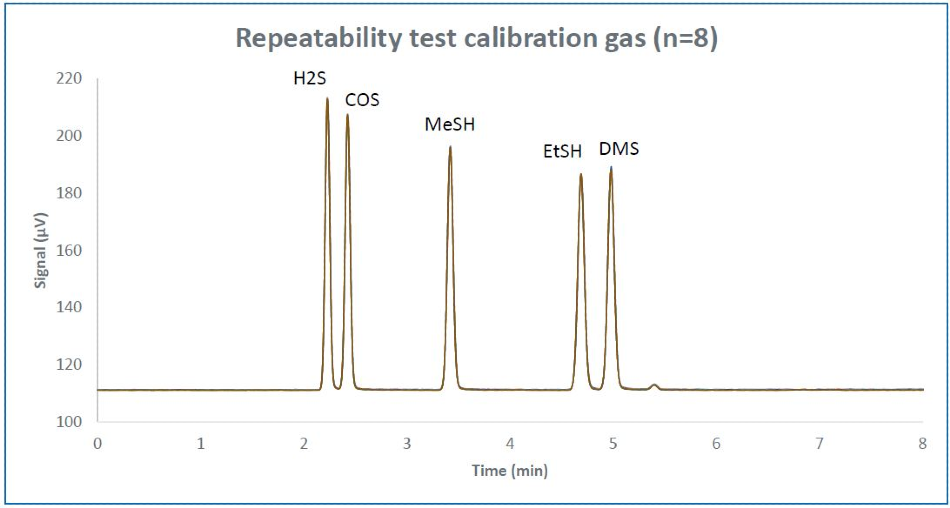
Figure 3. Repeatability overlay of 8 consecutive runs of calibration gas diluted to ~500 ppb.
Table 1. Retention time repeatability of 8 consecutive runs of calibration gas diluted to ~500 ppb.
| Run |
Retention time (minutes) |
Hydrogen
sulfide |
Carbonyl
sulfide |
Methyl
Mercaptane |
Ethyl
Mercaptane |
DMS |
| 1 |
2.232 |
2.428 |
3.423 |
4.692 |
4.982 |
| 2 |
2.231 |
2.426 |
3.422 |
4.690 |
4.981 |
| 3 |
2.230 |
2.426 |
3.421 |
4.690 |
4.981 |
| 4 |
2.229 |
2.425 |
3.420 |
4.689 |
4.979 |
| 5 |
2.230 |
2.426 |
3.420 |
4.690 |
4.980 |
| 6 |
2.230 |
2.426 |
3.421 |
4.690 |
4.980 |
| 7 |
2.231 |
2.426 |
3.421 |
4.690 |
4.981 |
| 8 |
2.230 |
2.425 |
3.420 |
4.689 |
4.980 |
| Average |
2.230 |
2.426 |
3.421 |
4.690 |
4.981 |
| MIN |
2.229 |
2.425 |
3.420 |
4.689 |
4.979 |
| MAX |
2.232 |
2.428 |
3.423 |
4.692 |
4.982 |
| stdev |
0.0009 |
0.0009 |
0.0011 |
0.0009 |
0.0009 |
| RSD |
0.04% |
0.04% |
0.03% |
0.02% |
0.02% |
Concentration Repeatability
As stated by ASTM D5504-12, concentration repeatability is calculated for 8 runs consecutively for a calibration standard blend diluted to approximately 500 ppb single peak. Repeatability is contrasted with the method’s statement on precision. Highly accurate repeatability values are acquired (Table 2).
The repeatability standard deviation collected on the analyzer is contrasted with the values given in the precision statement. The analyzer strongly correlates to the precision statement for carbonyl sulfide, methyl mercaptane, and hydrogen sulfide.
Table 2. Repeatability of a standard blend by GSV introduction (500 ppb).
| Run |
Concentration (ppm) |
Hydrogen
sulfide |
Carbonyl
sulfide |
Methyl
Mercaptane |
Ethyl
Mercaptane |
DMS |
| 1 |
0.471 |
0.455 |
0.456 |
0.482 |
0.476 |
| 2 |
0.466 |
0.449 |
0.455 |
0.478 |
0.467 |
| 3 |
0.469 |
0.449 |
0.453 |
0.475 |
0.470 |
| 4 |
0.471 |
0.454 |
0.454 |
0.476 |
0.470 |
| 5 |
0.473 |
0.454 |
0.455 |
0.479 |
0.470 |
| 6 |
0.466 |
0.451 |
0.455 |
0.479 |
0.472 |
| 7 |
0.468 |
0.450 |
0.456 |
0.480 |
0.471 |
| 8 |
0.471 |
0.456 |
0.452 |
0.483 |
0.472A |
| Average |
0.469 |
0.452 |
0.455 |
0.479 |
0.471 |
| MIN |
0.466 |
0.449 |
0.452 |
0.475 |
0.467 |
| MAX |
0.473 |
0.456 |
0.456 |
0.483 |
0.476 |
| RSD |
0.52% |
0.60% |
0.33% |
0.55% |
0.58% |
repelatability
stdev method |
0.0024 |
0.0027 |
0.0015 |
0.0026 |
0.0027 |
repelatability
stdev method |
0.10 |
0.06 |
0.10 |
- |
- |
Linearity
The analyzer’s linearity response is validated by producing dynamic dilutions of a certified calibration gas. The dilutions are prepared through the combination of the dilution gas (pure nitrogen) and calibration gas (sulfur compounds in nitrogen) utilizing two individual mass flow controllers.
Concentrations of approximately 10 ppm down to 10 ppb have been produced and investigated on the AC D5504 Ultra Low system. Calibration lines have been configured for carbonyl sulfide (COS), hydrogen sulfide (H2S), ethyl mercaptane (EtSH), DMS, and methyl mercaptane. A linearity correlation of less than 0.9999 is noted in all calibration lines.

Figure 4. Linearity Plot H2S.

Figure 5. Linearity Plot COS.

Figure 6. Linearity Plot MeSH.

Figure 7. Overlay diluted Calibration gas.
Detectability
The detection limit is determined using the following formula. The calculations are based on a calibration gas diluted to approximately 50 ppb. The results are outlined in Table 3.
To validate the detectability of the AC D5504 Ultra low system calculated, the calibration gas is diluted (in the same manner as the linearity measurement) down to 5 ppb level and investigated on the analyzer.

Where:
LOD = Limit of detection (ppm mol)
c = Concentration of component of interest (ppm mol)
N = Noise (peak to peak) (µV)
A = Area of peak of interest (µV*s)
W = Width of peak at half height (minutes)
Table 3. Detection limit calculation.
| Component |
Noise (µV) |
Area (µV*s) |
Conc. (ppb) |
Width (min) |
LDL (ppb) |
| Hydrogen sulfide |
0.2 |
33.4 |
49 |
0.0542 |
2.9 |
| Carbonyl sulfide |
0.2 |
34.6 |
48 |
0.0580 |
2.5 |
| Methanethiol |
0.2 |
34.1 |
48 |
0.0656 |
3.3 |
| Ethanethiol |
0.2 |
34.8 |
50 |
0.0785 |
4.1 |
| Dimethyl Sulfide |
0.2 |
37.5 |
50 |
0.0792 |
3.8 |

Figure 8. Overlay low level diluted Calibration gas.
Equimolarity
As the SCD is an equimolar detector, it is assumed that all sulfur compounds will create the same response as sulfur.
The response factors for each of the calibration components in the calibration gas were determined and are presented in Table 4. The response factor of each individual sulfur compound is within 5% of the response factor for hydrogen sulfide.
Table 4. Response factor calculation at 0.50 ppm Sulfur level.
| Concentration |
Concentration
ppm |
Average
Area |
RF |
Deviation
RF to H2S |
| Hydrogen Sulfide |
0.49 |
343 |
0.001429 |
0.00% |
| Carbonyl Sulfide |
0.48 |
353 |
0.001359 |
-4.92% |
| Methyl Mercaptan |
0.48 |
350 |
0.001370 |
-4.11% |
| Ethyl Mercaptan |
0.50 |
351 |
0.001425 |
-0.29% |
| Dimethyl Sulfide |
0.50 |
366 |
0.001368 |
-4.27% |
Natural Gas
The Ultra-low D5504 system is used to analyze a sample of natural gas (acquired from the local distribution network). The primary peak is THT (Tetra Hydro Thiophene) which is introduced to the natural gas network by the supplier as an odor component at approximately 18 mg/m3.
The identified concentration is 4.55 ppm mol THT, which is in line with 16.99 mg/m3. Observing the baseline more closely, a range of sulfur compounds (such as DMS) are identified at single-digit ppb levels.

Figure 9. THT in Natural Gas sample with Zoomed chromatogram.
Conclusion
The AC D5504 Ultra Low analyzer is a resolute solution for the precise quantification of sulfur compounds in gaseous fuels and natural gas.
Its efficacy not only adheres to the ASTM D5504 standards but also exceeds them, and ensures that the highest quality data can be employed to predict the effects of sulfur compounds in gaseous fuels and natural gas.
Combined with the exclusive AC SeNse detector, already well established for its ruggedness and stability, the AC D5504 Ultra Low is highly robust and simple to use in everyday environments.
The AC Ultra Low D5504 analyzer offers low detection levels (down to approximately 5 ppb), and excellent stability, equimolarity, repeatability, and recovery values each time.
Table 5. AC ASTM D5504 ordering guide.
| Ordering information |
Description |
| CCG6104A |
ASTM D5504 Ultra Low Sulfur System, 120V 7890GC |
| CCG6104C |
ASTM D5504 Ultra Low Sulfur System, 230V 7890GC |
| CCG6104.100 |
Kit, Spares & Consumables for ASTM D5504 Ultra Low Sulfur analyzer, on 7890GC |
| 99.10.040 |
Calibration gas, Sulfur, without regulator (H2S, COS, MeSH, EtSH, DMS –10 ppm Mol) |
| 99.10.014 |
Pressure regulator, for Sulfurcalibration gas, inert |
| CCG6199 |
Permeation Device, build-in for Sulfuranalyzeron 7890GC |

This information has been sourced, reviewed and adapted from materials provided by PAC L.P.
For more information on this source, please visit PAC L.P.