
Thermo Scientific™ Evolution™ UV-Visible Spectrophotometers with Thermo Scientific™ INSIGHT™ Software provide a reliable, simple-to-use solution for pharmacopeia compliance support.
The EP and USP both call out the following for parameters for performance verification (PV). They test absorbance accuracy, wavelength accuracy, resolution, and stray light.
Thermo Fisher Scientific has prepared an outline of options that are available for utilization in PV testing, as the choice of standards can be a challenge. There are two kits available which include the essential standards for PV testing to help simplify the selection process. Standards can also be purchased separately.
In this article, the individual needs for each test will be described and guidance on the selection of standards to complete the PV testing will be offered.
Table 1.
| Part Number |
Standards Kit |
Performance
Verification Test |
Reference Materials |
| 222-327200 |
USP and EP UV Standards Set |
Wavelength Accuracy |
Calibrated holmium oxide solution for wavelength accuracy from 241 nm to 641 nm |
| |
|
Photometric Accuracy |
Calibrated 60 mg/L, 80 mg/L and 140 mg/L potassium dichromate with blank: UV absorbance accuracy to 2A |
| |
|
Stray Light |
Certified potassium chloride, sodium iodide, and sodium nitrate for stray light measurements |
| |
|
Resolution |
Certified toluene in hexane solution with hexane blank for resolution (spectral bandwidth) testing |
| 222-327300 |
USP and EP Vis Standards Set |
Wavelength Accuracy |
Didymium glass filter |
| |
|
Photometric Accuracy |
4 neutral density filters calibrated for absorbance at 440, 465, 546.1, 590 and 635 nm. Absorbance values approximately 0.5, 1.0, 1.5 and 2.0 |
Photometric (Absorbance) Accuracy
For the wavelength and absorbance ranges required, it is necessary to verify the absorbance accuracy of a system across its intended operational range.
Operational Range = the absorbance range where you measure samples in your lab, measured near the wavelength where you measure the samples.
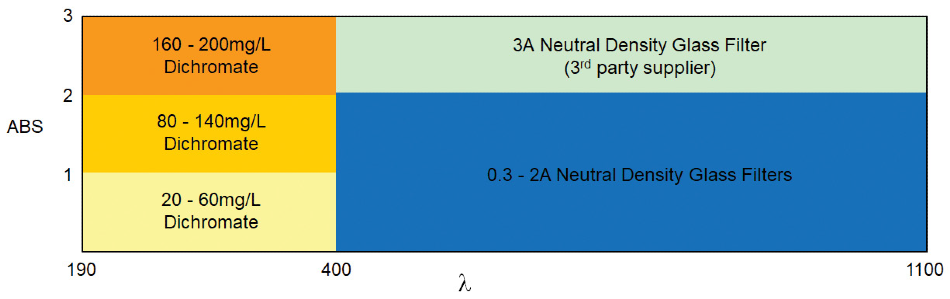
What is required:
- Choose at least three standards at different absorbance levels which span the measured operational range
- For EP, it is recommended not to test >2.00A
- For USP, one standard has to be in the 0 – 2.00A region. If measuring >2.00A, standards evaluating this region must be selected.
Table 2.
Potassium Dichromate
solutions (K2Cr2O7) |
Concentration
(mg/L) |
Part Number |
| USP and EP UV Standards Set* |
60 mg/L, 80 mg/L and 140 mg/L |
222-327200 |
UV Photometric Accuracy Stds
for USP-2015 to 2A* |
60 mg/L and 140 mg/L |
840-288900 |
| Potassium Dichromate |
100 mg/L |
840-288300 |
| Potassium Dichromate |
120 mg/L |
840-288400 |
| Potassium Dichromate |
160 mg/L |
840-288600 |
| Potassium Dichromate |
180 mg/L |
840-288700 |
| Potassium Dichromate |
200 mg/L |
840-288800 |
Potassium Dichromate 5 Stds kit
(from 0.1A to 1.5A) |
20 mg/L – 100 mg/L |
9423UV95200E |
*Recommended
For UV wavelength absorbance (<400 nm)
- Choose at least three potassium dichromate standards
- <1A 20-60 mg/L K2Cr2O7
- 1A – 2A samples: add an 80 – 140 mg/L K2Cr2O7
- 2A – 3A samples: add a 160 – 200 mg/L K2Cr2O7
For visible wavelength absorbance (>400 nm)
- Choose at least three neutral density glass filters
- One between 0-2.00A
- At least two more additional filters depending on the operational range
Table 3.
| Neutral Density Filters |
Part Number |
| USP and EP Vis Standards Set* |
222-327300 |
| Qualification Filter Kit for UV-Vis |
840-326000 |
Photometric accuracy and linearity test kit
Calibrated ND filters (6 filters from 0.3A to 2A) |
840-253000 |
*Recommended
Wavelength Accuracy
Wavelength accuracy and precision are to be determined across the operational range.
Operation range = the wavelengths where samples are measured in your lab.
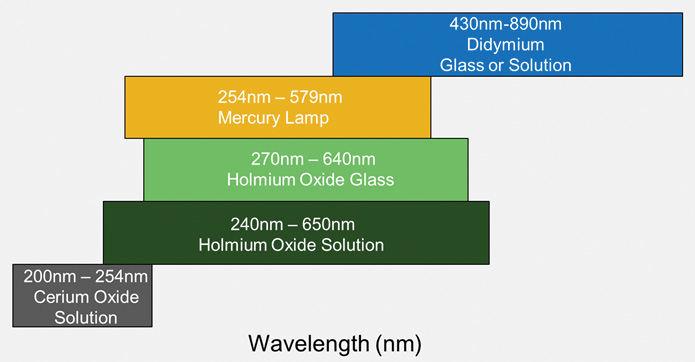
What is required:
- Either holmium oxide or a mercury lamp
- Didymium oxide is also required if measuring at >650 nm
- Cerium oxide is recommended if measuring in the far UV
Table 4.
| Products containing Wavelength Standards |
Part Number |
| USP and EP UV Standards Set* (Holmium oxide solution) |
222-327200 |
| USP and EP Vis Standards Set* (Didymium glass filter) |
222-327300 |
Qualification Filter Kit for UV-Vis
(Holmium glass and didymium glass filters) |
840-326000 |
| Wavelength Standard – Didymium Glass |
840-325800 |
| Wavelength Standards, Holmium, and Didymium |
840-325700 |
| Cerium Oxide Standard |
222-327400 |
*Recommended
Mercury Lamp Options for Wavelength Accuracy
Mercury vapor lamps have numerous benefits over other “standards” employed in PV testing. These lamps produce atomic emission lines that do not alter, and they are extremely well characterized. All low pressure mercury lamps generate emission lines at exactly the same wavelengths.
Mercury vapor emission spectra are what other standards, like holmium oxide solutions, are calibrated against as a standard and it is never necessary to re-calibrate a mercury lamp. The USP states atomic line spectra as its first wavelength accuracy choice for wavelength accuracy testing, stating,
“This procedure is described as the primary application because the emission lines produced from a discharge lamp are characteristic of the source element and, as a fundamental physical standard; these wavelengths have been measured with an uncertainty of not more than ±0.01 nm.”
The EP also calls out atomic line spectra for this purpose, “Control the wavelength accuracy of an appropriate number of peaks in the intended spectral range… by measuring the emission from a light source for the verification of emission-line position.”
An optional factory-installed mercury lamp is available with The Thermo Scientific™ Evolution™ 350 UV-Vis Spectrophotometer. For the Evolution 200 series instruments, a mercury lamp accessory is also available.
Table 5.
| Instrument |
Mercury Lamp Accessory Part Number |
| Evolution 201, 220, 260 |
840-211300 |
| Evolution 350 |
840-310900 |
Resolution
What is required: Establish the resolution of the spectrometer in the UV region by utilizing a 0.020% (v/v) solution of toluene in n-hexane with n-hexane as the reference.
Table 6.
| Reference Materials |
Standard |
Part Number |
| Certified toluene in hexane solution with hexane blank |
USP and EP UV Standards Set |
222-327200 |
| Certified toluene in hexane solution with hexane blank |
Resolution Standard – Toluene in Hexane |
222-226600 |
Stray Light
As part of performance qualification (PQ), the level of stray light must be monitored at the appropriate wavelength(s). Analysts can measure the absorbance of the filters specified against the appropriate reference, and record the maximum absorbance value.
What you will need based on your operational wavelength:
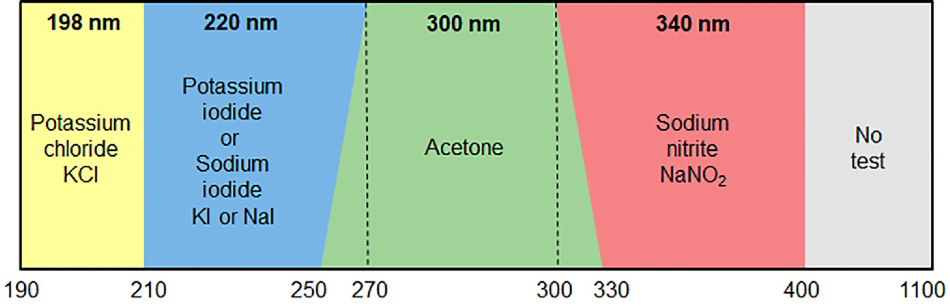
Table 7.
| Reference Materials |
Standard |
Part Number |
| KCl, NaI, and NaNO2 |
USP and EP UV Standards Set |
222-327200 |
KCl
190 – 210 nm
Measure at 198 nm |
Stray light filter, potassium chloride, KCI |
9423UV95520E |
Nal
210 – 270 nm
Measure at 220 nm |
Stray light filter, sodium iodine, NaI |
9423UV95500E |
Acetone
250 – 330 nm
Measure at 300 nm |
Acetone stray light standard and blank |
840-284400 |
NaNO2
300 – 400 nm
Measure at 340 nm |
Stray light standard, sodium nitrate |
222-226500 |
Thermo Scientific Evolution UV-Vis Spectrophotometers
When meeting today’s pharmacopeia standards is a top priority, choosing a UV-Visible spectrophotometer should be simple.
Each Evolution UV-Vis Spectrophotometer is available in bundle packages with different standards kit choices:
- Thermo Scientific™ INSIGHT™ Security software for support of 21 CFR Part 11 Compliance
- A choice of an Evolution UV-Vis spectrophotometer
- The choice of performance verification standards kit:
- Qualification Filter Kit for UV-Vis – Contains the standards required for general instrument qualification. Includes some standards which are used for pharma PV testing, but additional standards will be needed for total compliance
- USP and EP UV and Vis Standards Sets – Contains all of the standards required to meet the general EP and USP testing requirements
- Thermo Scientific™ Validator™ documents for initial and re-qualification for the instrument’s lifetime
The versatile Evolution 200 series and Evolution 350 UV-Vis Spectrophotometers provide:
- INSIGHT 2 software with enhanced compliance features
- Precision monochromator drive which supplies high accuracy and quick scanning
- 1 nm fixed or selectable bandwidths with double-beam optics
Table 8.
| Description |
Part Number |
| Evolution 201 Computer Control UV-Vis |
840-210800 |
| Evolution 220 Computer Control UV-Vis |
840-210600 |
| Evolution 260 Bio Computer Control UV-Vis |
840-211000 |
| Evolution 350 Computer Control UV-Vis |
840-310800 |
| Evolution 350 with Mercury Lamp |
912A0959 |
Selecting the Evolution UV-Vis Model Which Meets Your Requirements
The Evolution 201 spectrophotometer has a 1.0 nm spectral bandwidth for high-resolution data in basic research applications and routine quality control.
With a selectable bandwidth option for a wider variety of applications, the Evolution 220 spectrophotometer enhances the versatility of a system. This model features applications focused beam geometry which is optimized for employment with integrating fiber optic probe, sphere accessories (diffuse transmittance/reflectance), and microcells.
For higher productivity in your life science laboratory to the Evolution 220 platform, the Evolution 260 Bio spectrophotometer adds the convenience of pre-programmed bio applications.

The Evolution 350 spectrophotometer is a high-performance solution that has an extra-large sample compartment for expanded accessory options and a parallel beam design. This model includes a mercury lamp option for supporting pharmacopeia wavelength and bandwidth accuracy testing.

Validated Installation
The unity lab services professionals at Thermo Fisher Scientific can handle your installation, providing:
- Peace of mind – users are trained on equipment operation and routine care, while equipment will be installed and configured to their manufacturing specifications ensuring the best performance.
- Improved productivity – accurate installation scheduled at your convenience, optimizing both user performance and equipment.
Table 9.
| Description |
Part Number |
| Evolution 350 Validated Installation |
701-680300 |
Validated installation includes:
- User orientation and instrument familiarization
- Installation Qualification and Operational Qualification
- On-site installation of instrument
- All engineer travel charges and labor for installation
Does not include instrument Performance Qualification (PQ)
Standards Rental Options
Thermo Fisher Scientific also provides the option to include the rental of standards for performance verification with service. Standards rental may be added to the service options below.
Table 10.
| Description |
Part Number |
| USP Standards Rental with Validated Installation |
701-055587 |
| USP Standards Rental with Recertification Contract |
701-055588 |
| USP Standards Rental with Performance Verification |
701-173000 |
Performance Verification
Table 11.
| Description |
Part Number |
Performance verification for Evolution Spectrophotometers
includes Validator qualification materials |
701-023300 |
Annual Recertification
Table 12.
| Description |
Part Number |
| Optional annual recertification with a contract for Evolution Spectrophotometers* |
701-509300 |
* Models include Evolution Array, Evolution 60S, Evolution 200, Evolution 300 or Evolution 600 Spectrophotometers

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific – Materials & Structural Analysis.
For more information on this source, please visit Thermo Fisher Scientific – Materials & Structural Analysis.