In November 1996, the ICH Q1B Guideline was finalized. This guidance details the principles needed to assess the light sensitivity and stability of new drug products and substances.
Image Credit: Jordi Labs
Q1B has been adopted, published and implemented by noteworthy institutions. These include:
- FDA (US)
- EC (Europe)
- Swissmedic (Switzerland)
- Health Canada (Canada)
- MHLW/PMDA (Japan)
Primarily, this guideline tackles the generation of photostability information for submission in Registration Applications for new molecular entities and associated drug products.
A systematic approach to photostability testing is recommended in the guideline, in addition to light sources and procedures. The image below shows a suggested decision flowchart for photostability testing.
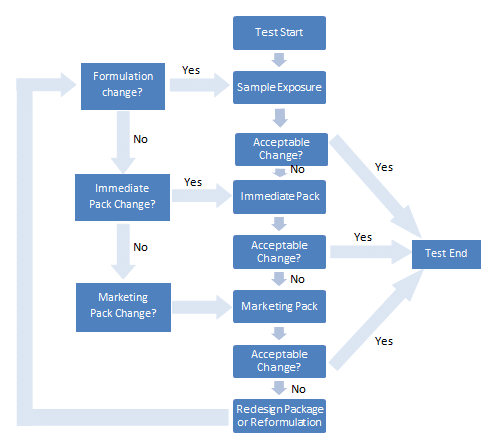
Image Credit: Jordi Labs
The Q1B Guideline supplies the recommended testing for drug products and substances, including analysis and preparation of samples, and techniques to be used for evaluating results. Photostability testing should include forced degradation testing and confirmatory testing for drug substances.
For drug products, studies should be performed in a sequential manner. These should begin with testing the fully exposed product then progressing as needed to the product in the immediate pack and then in the marketing package.
References and Further Reading

This information has been sourced, reviewed and adapted from materials provided by Jordi Labs.
For more information on this source, please visit Jordi Labs.