Plastic litter has emerged as a pollutant of concern in the oceans around the world over the past few decades. Defined as plastic litter that is smaller than 5 mm, microplastic is thought to be the most common form of marine debris.1,2
Microplastics include both small manufactured items like beads and fibers, known as primary microplastics, plus items that result from fragmentation of larger plastic items by a combination of chemical, physical and biological processes and include fragments, foams and films, known as secondary microplastics.
Plastic marine debris usually starts as land-derived waste and then enters the coastal ocean and estuaries.3 Microplastics distribute throughout the water after entering the marine environment; this is through the help of turbulence, tides and ocean currents, dispersing them throughout the water column and across the oceans.4
The high biological productivity and rich ecosystems found in coastal environments suggest that there are more frequent biological interactions with microplastics in the coastal region than in the open ocean.5
Although in estuarine and coastal waters the likelihood of plastic accumulation is bigger, there are not many studies focusing on microplastics in these regions.6 It has become vital to expand the capabilities of research laboratories to routinely analyze the chemical composition of candidate microplastics from environmental samples due to the explosion of microplastics research.
Usually, visual inspection is utilized to isolate microplastics from field-collected samples, but this can result in the accidental exclusion of plastic pieces and misclassification of microplastics.
As they can confirm manual microplastic designation through polymer identification, spectroscopic methods are vital. These techniques can help to establish where material may have come from and could identify additives that themselves could pose negative biological effects.
Polymers, and by extension plastics, usually give a strong Raman signal. The Raman spectra of bulk polyethylene and polypropylene materials measured with 1064 nm excitation can be seen in Figure 1. The plastics can be clearly distinguished based on their spectral features.
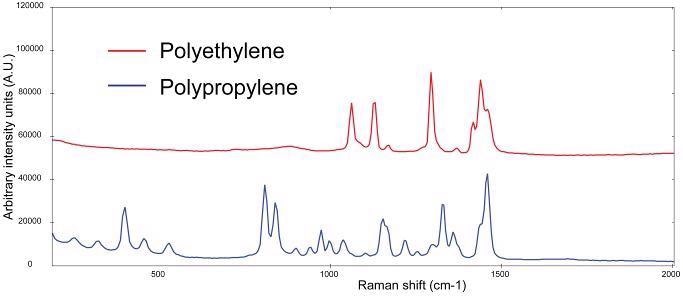
Figure 1. Raman spectra of polypropylene (blue) and polyethylene (red, spectra manually offset for clarification). Image Credit: B&W Tek
A reference library can be created for easy identification of unknown microplastic material and there are also commercial libraries available. Raman provides easier sampling of small (<100 µm) particles than FTIR, although Fourier transform infrared (FTIR) spectroscopy is another method that is frequently used for the identification of microplastics.
Raman spectrometers are more portable than FTIR systems, so portable Raman analysis may be utilized at the location of sample preparation. In this article, the utilization of portable Raman microscopy for the identification of microplastics recovered from surface estuary waters will be explored.

Image Credit: B&W Tek
Experiment
By performing 5 min tows with a ring plankton net (1 m diameter, 200 μm nitex mesh) fitted with a flow meter, water samples were gathered from the surface water of the Delaware Bay (USA). Seven samples were then transferred to glass jars and fixed with 4% formaldehyde.
The total sample was size-fractionated on stainless steel sieves (5,000; 1,000; and 300 μm). Wet peroxide oxidation and density separation processes were utilized after drying the samples overnight at 90 °C. This was done to isolate microplastic from digested organic material in the two smaller size-fractionated samples.8
Next, the microplastics were gathered onto 200 μm nitex mesh and folded in aluminum foil to dry. Microplastics from these samples were enumerated using manual examination under a stereomicroscope in a laminar flow hood and each piece was assigned a plastic-type (i.e., bead, fragment, foam, fiber, rubber, film).
Plastic identification using Raman spectroscopy followed categorization and enumeration. For all measurements, an i-Raman® EX portable Raman system with a 1064 nm laser excitation was employed; the specifications can be seen in Table 1.
Table 1. i-Raman® EX Specifications. Source: B&W Tek
| . |
. |
| Laser wavelength |
1064 nm |
| Maximum laser power at probe |
330 mW |
| Spectral range |
100-2500 cm-1 |
| Detector type |
InGaAs TE-cooled to -15 °C |
The 1064 nm laser excitation is needed to mitigate the fluorescence, which is usually produced by colored microplastic samples upon excitation with a 785 nm laser. In order to image the microplastics, a portable video microscope setup with an objective lens of 50× magnification (9.15 mm working distance, 42 µm spot size) was employed.
BWSpec® software was employed for gathering the data. Integration times ranged from 30s-3 minutes and in order to avoid sample burning, laser power was kept at less than 50% of the maximum laser power (<165 mW).
BWID® software was utilized to make the identification of the microplastics against a reference library of plastics spectra. The spectra were intensity corrected against a NIST 2244 Raman intensity correction standard, but no other preprocessing was applied to the spectra.
Results
The i-Raman EX system was used to analyze multiple microplastics samples of different sizes and types. Figure 2a shows the photo of a large blue microplastic fragment, the diameter of this fragment is ~4.5 mm, which is on the higher end of the size range for a particle to be designated as a microplastic.
The sample is likely a secondary microplastic because of the irregular shape of the particle. The Raman spectrum collected from the blue fragment is shown in Figure 2b. BWID software uses a calculated hit quality index (HQI) to compare the acquired spectrum of the unknown sample to a library of reference materials.
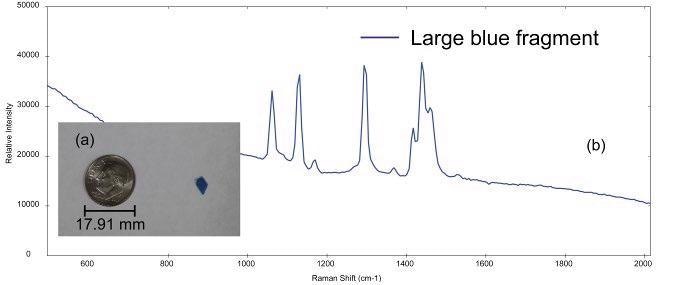
Figure 2. (a) Small blue plastic fragment (dime for comparison) and (b) Raman spectrum acquired from the sample. Image Credit: B&W Tek
The HQI is a correlation coefficient that looks at how similar the sample spectrum is to the reference spectrum. Spectral library search results are ranked from a HQI of 0 (worst match) to a HQI of 100 (best match). For the calculation, a first derivative is applied to the spectra.
There are a number of spectral libraries available for utilization with BWID and it also supports custom library building. BWID matched the blue fragment in Figure 2a to a reference spectrum of polyethylene (PE) with a calculated HQI of 95.7 (Figure 3); this showed a strong match.
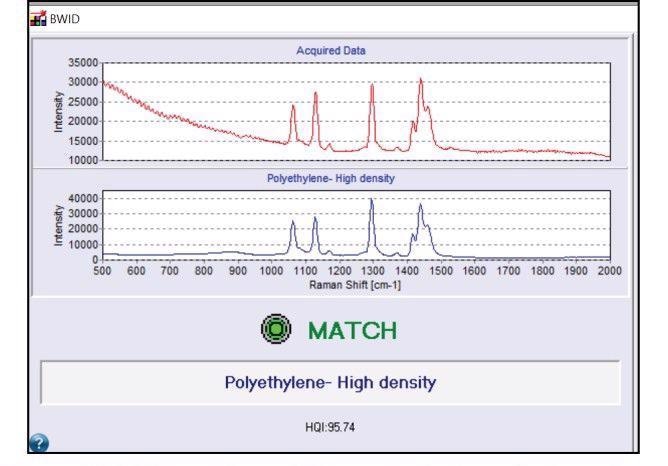
Figure 3. BWID match result for polyethylene. Red spectrum is the acquired sample spectrum from Figure 2. Blue spectrum is the reference spectrum of polyethylene. Image Credit: B&W Tek
Other plastic materials, like polypropylene (PP) and polystyrene (PS), were also identified.
Figure 4a exhibits the Raman spectrum collected from a small, spherical bead (photomicrograph shown in Figure 4b). This bead is probably a primary microplastic. BWID matched the spectrum to a reference spectrum of polystyrene with an HQI of 98.2.
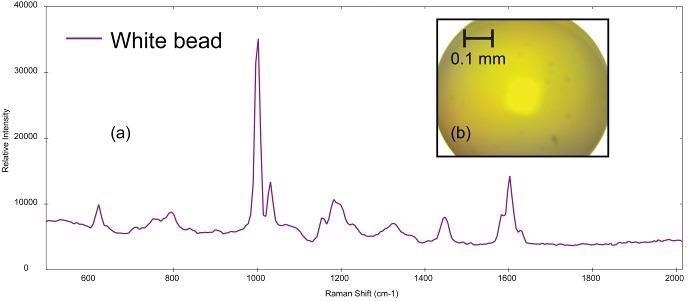
Figure 4. (a) Raman spectrum of polystyrene collected from (b) polystyrene bead (image not true color). Image Credit: B&W Tek
Fibers are a vital subgroup of microplastic particles and during the production process, they can be shed from synthetic garments and other plastic textiles. They can also be shed through wastewater during routine home maintenance and synthetic fishing lines are also a key source of microfiber pollution.
Figure 5a shows the Raman spectrum gathered from a thin, teal fiber (photomicrograph shown in Figure 5b). BWID matches a reference spectrum of polypropylene to the Raman spectrum of the sample, with a calculated HQI of 74.9. Although this value may seem low, upon further examination, there are some more peaks in the sample spectrum, which cannot be attributed to polypropylene.
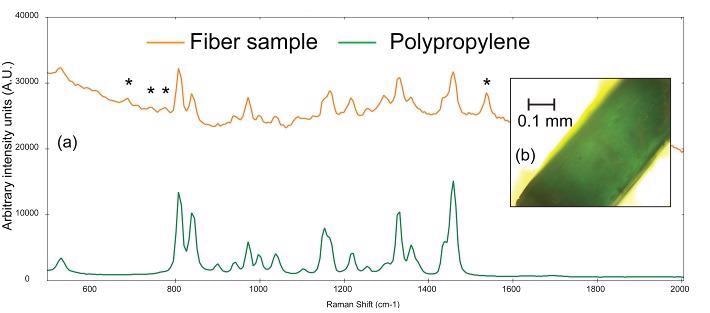
Figure 5. (a) Raman spectra of a teal fiber (orange) compared to a reference spectrum of polypropylene (green) and (b) photomicrograph of teal fiber. The asterisks denote peaks that can be attributed to the colorant used in the plastic. Image Credit: B&W Tek
The set of weak peaks from 670-790 cm-1 and the peak at ~1537 cm-1 in the sample spectrum are consistent with the Raman spectrum of chlorinated copper phthalocyanine green pigment.9
This is helpful information for establishing the origin of a sample even though the colorant used in the plastic is not generally a key research question. The summary of the microplastic analysis from the Raman spectra is shown in Table 2. All of the materials were identified as polypropylene, polyethylene, or polystyrene.
Table 2. Summary of BWID results. Source: B&W Tek
| Match result |
Number of samples identified |
| PE |
11 |
| PP |
4 |
| PS |
2 |
| inconclusive |
5 |
A number of samples had inconclusive results; most of these samples are black microplastic samples that absorb both the scattered radiation and exciting radiation, making their identification with Raman spectroscopy extremely challenging.
Another further limitation observed was the fragility of the microfibers. As higher laser powers may cause distortion and burning of the sample, the laser power applied to fibers must be kept low (~10% of the maximum laser power).
Conclusions
Their robust characterization of microplastics in our marine environments will be a crucial research topic for years to come because their presence represents a looming threat to our environment.
Raman microscopy is an effective tool that can be used to identify these microplastics unambiguously. In order to mitigate fluorescence from the dyes used in the plastics, the use of near-infrared excitation is crucial. For the simple identification of plastic material, software correlation coefficient algorithms are helpful.
References
- K.L. Law, Annu. Rev. Mar. Sci. 9, 205-229 (2017).
- T.S. Galloway, M. Cole and C. Lewis, Nat. Ecol. Evol. 1 (2017).
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R Siegler, M. Perryman, A. Andrady, R. Narayan, and K. L. Law, Science. 347, 768-771 (2015).
- R. C. Hale, M.E. Seeley, M.J. La Guardia, L. Mai and E.Y. Zeng, J. Geophys. Res. Oceans. 125 (2020).
- J. R. Clark, M. Cole, P. K. Lindeque, E. Fileman, J. Blackford, C. Lewis, T. M. Lenton, and T. S. Galloway, Front. Ecol. Environ. 14, 317-324 (2016).
- P. Vermeiren, C.C. Muñoz, and K. Ikejima, Mar. Pollut. Bull. 113, 7-16 (2016).
- J.H. Cohen, A.M. Internicola, R.A. Mason, and T. Kukulka, Environ. Sci. Technol. 53, 14204−14211 (2019).
- J. Masura, J. Baker, G. Foster, and C. Arthur, “Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles” in Waters and Sediments; NOAA Technical Memorandum NOSOR&R-48, National Oceanic and Atmospheric Administration: Washington, DC, 2015.
- A. Duran, M. L. Franquelo, M. A. Centeno, T. Espejoc, and J. L. Perez-Rodrigueza, J. Raman Spectrosc. 42, 48–55 (2011).
Acknowledgments
Produced from materials originally authored by Jonathan H. Cohen and Taylor Hoffman from University of Delaware School of Marine Science and Kristen Frano from B&W Tek.

This information has been sourced, reviewed and adapted from materials provided by B&W Tek.
For more information on this source, please visit B&W Tek.