Sponsored by MerckJan 4 2021
Carbon nanotubes are materials that have extraordinary properties which are useful across a variety of new, state-of-the-art applications in sensors, printed electronics, e-readers, flexible displays, energy storage medical treatment and more. Since their discovery in 1991 by Ijima1, SWNTs have influenced a significant amount of activity in both research and industry across the world.
Furthermore, they and have stimulated considerable investment in manufacturing methods, characterization and application development. This text describes the physico-chemical nature and characterization of single-wall carbon nanotubes (SWNTs) and the status of their commercialization. Multi-wall carbon nanotubes (MWNTs) are outlined on their own merit via MWNT Spotlight.
Structure of Carbon Nanotubes
Like fullerenes, SWNTs are an allotrope of sp2 hybridized carbon. The structure can be regarded as a cylindrical tube made up of 6-membered carbon rings, as in graphite. The cylindrical tubes can have one or both ends capped with a fullerene structure or hemisphere of the buckyball.
An understanding of SWNT structure necessitates an acquaintance with the theory of nanotube chirality, since the chirality of a SWNT commands many of its properties. The chirality map, shown in Figure 1, has been designed as a tool for comprehending chirality and its implications.
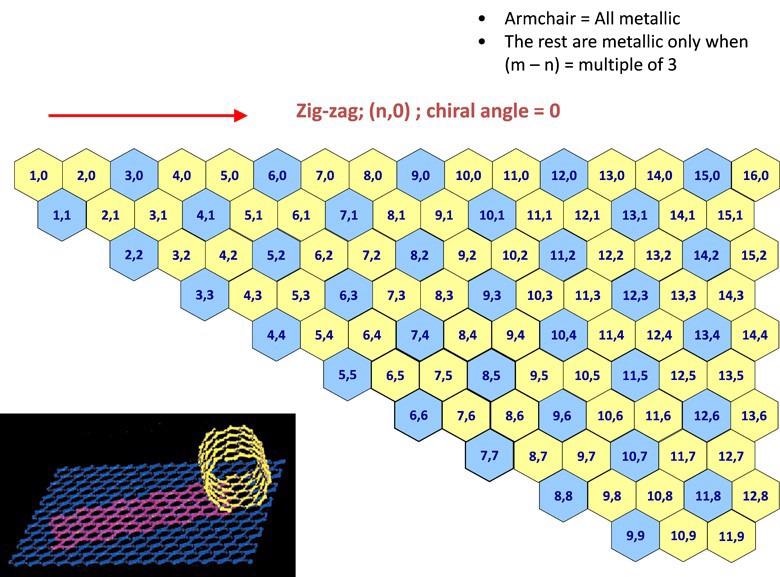
Figure 1. A graphic displaying a chirality map which shows the various types of SWNTs that can be formed. The properties are governed by the way in which they are rolled as shown in the inset. The SWNT will be metallic in the armchair configuration, or when m-n is a multiple of 3. Image Credit: Merck Millipore Sigma
A SWNT can be visualized as a sheet of graphene (one atom thick) rolled into a tube as illustrated in Figure 1. The chirality outlines both the diameter and the orientation to which the sheet is rolled. Each SWNT on the chirality map is identified by two integers, (n,m). Chirality designates several of an individual SWNT’s properties. For instance, SWNT illustrated on the chirality map in blue are naturally metallic. These are tubes where n=m (armchair) or n – m = 3i, (where i is any integer). Those represented as yellow are semiconducting, displaying various band gaps depending on the chiral vector’s length.
Unique Properties of SWNT
Mechanical: Individual SWNTs are considerably stronger than steel. Values calculated for tensile strength of SWNT are ~100 times greater than steel at 1/16th the weight. The greatest measured value is around half of the theoretical strength anticipated, the difference potentially being due to defects in the structure.4
Electrical: The current carrying capacities of individual SWNTs are 109 amp.cm-2; more than those of gold or copper,5 and semiconducting species demonstrate electron mobility greater than silicon.
Optical: With each chirality exhibiting its own characteristic absorption and fluorescence spectrum, SWNTs have a clear-cut optical absorption and fluorescence response.
Thermal: Room temperature thermal conductivity of an individual nanotube may be comparable to that of diamond or in-plane graphite, which is typically thought to display the greatest measured thermal conductivity of any known material at moderate temperatures.
Challenges of SWNTs
The widespread application of SWNTs has been somewhat limited by technical barriers in the areas of purity, selectivity and dispersibility. However, recent progress addresses these challenges.
Purity: The diversity of manufacturing processes used in SWNT production results in products which are contaminated to various degrees with residual catalyst and other forms of carbon. For several applications, additional purification processes are required to eliminate these contaminants to generate a product of an appropriate purity. More recently, commercial methods of synthesis that minimize the 'synthesized' impurities have become known.
Selectivity: As previously highlighted, SWNTs are a combination of tubes of different chiralities, some of which are semiconducting and some that are electrically conducting. It is preferrable for many applications to separate each type of tube, such as metallic from semiconducting and for some applications, tubes with distinct individual chirality.
Laboratory scale methods to attain an extremely high-degree of selectivity have been reported,2 and efforts to develop scalable separation processes are proving to be productive. Manufacturing processes such as the CoMoCAT® catalytic CVD process have demonstrated the capacity to offer a good degree of selectivity toward certain chiralities in SWNTs as synthesized, making the production of secondary purification processes considerably greater or even unnecessary.
Dispersibility: It can be difficult to disperse SWNTs, due in part to their tendency to form ropes or bundles due to Van der Waals attraction between the tubes. However, using aqueous solutions with the aid of suitable surfactants can disperse the SWNTs either as compact bundles or as individual tubes, or at low concentrations with suitable functionalization.
Exfoliation of bundles can be accomplished via sonication of aqueous solutions of SWNTs with the appearance of surface active molecules such as DNA, sodium deoxycholate and sodium cholate (Product No. 270911). To measure the degree of nanotube exfoliation acquired in a given dispersion, Tan and Resasco established the concept of resonance ratio from the optical absorbance spectrum.3 The area of the resonant band divided by the area of the non-resonant background in this ratio enables simplified comparison of results independent of the absolute absorption. Dispersants could then be ranked for their benefits using this parameter.
The dispersion of SWNTs in resins and thermoplastics is inhibited by a drastic build up in viscosity caused by the entanglement of the SWNTs bundles. Different proprietary technique exist to avoid this problem and new hybrid forms of SWNTs are in development to address this issue.
Synthesis of SWNTs
Several techniques have been utilized in the manufacture of SWNTs. These include carbon arc, laser ablation and CVD processes, either necessitating a gaseous catalyst as in the HIPCO® process or by utilizing a supported catalyst as in the CoMoCAT® process. The carbon arc process generates long tubes with diameters in the range 1.4 to 2.0nm, but carbon arc material has a great amount of impurities and for most applications extensive purification is necessary. The laser ablation process is used predominantly for research materials.
The CVD processes offer the best approach to the manufacture of larger SWNTs quantities, with perhaps the most scalable being the CoMoCAT® process which utilizes a fluidized bed reactor akin to those used in petroleum refining, albeit on a much smaller scale for the time being. The supported catalyst approach also offers the distinct ability to offer a substantial degree of chirality control throughout synthesis.
Characterization of SWNTs and Quality Assurance Parameters
As already discussed, the properties of carbon nanotubes differ with the chirality of individual SWNTs. Currently, all SWNTs are fabricated as a mixture of chiralities, the properties of the material is dependent the proportions of chiralities present. Numerous analytical methods have been employed to identify the structure of SWNT materials. These range from observational techniques such as AFM, SEM, STM and TEM and to spectroscopic techniques such as, photoluminescence (PL), Raman and UV-Vis-NIR.
In addition to these methods, X-ray diffraction has been utilized by Miyata et al. to determine the chirality assignments in the optical spectrum of SWNTs.8 Thermogravimetric analysis (TGA) has been employed liberally to identify the onset of oxidation, maximum oxidation rate and the mass of catalyst retained in the product. In some cases, it is possible to acquire a reasonable approximation of purity from the TGA curve.
TEM and SEM have been mostly used to assess SWNT purity. However, these cannot be relied on for any quantitative estimation of purity. Generally, a TEM or SEM image uses ~ 1pg of material over an area of 1 to 4 μm2 and it would therefore demand an analysis of multiple micrographs arbitrarily imaged throughout a macroscopic sample to acquire any meaningful results of the overall purity.
There are no algorithms appropriate for objectively determining the relative proportions of the various species seen in standard unpurified SWNT material. Therefore, while TEM and SEM can offer satisfactory information on the structure of the product, caution must be taken using these systems, considering them to act only as qualitative indicators of purity.
There are three relatively simple methods that are frequently that can be used in conjunction to make sure that consistent high quality SWNTs are generated. Raman spectrum can be used as a rough purity fingerprint for quality assurance purposes. TGA provides a measure of leftover catalyst and, as will be discussed later on, a reasonable measure of the SWNTs content. In order to ensure consistent chirality control optical absorption measurements in the UV-Vis-NIR region can be used.
Raman spectroscopy
Analysis by Raman spectroscopy has been used broadly for identifying both the complete combination of chiralities present in the SWNTs material and for evaluating purity. There are three areas of the Raman spectrum of significant interest for SWNTs. The radial breath mode (RBM) from approximately 120 to 300 cm-1 is distinct to SWNTs and can be utilized to identify tube diameter using the equation:
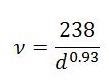
where d is the SWNT’s diameter in nm and ν is the wave number in cm-1
It is crucial to note that to acquire an accurate and complete picture of the chiralities, multiple lasers of various excitation frequency must be used. Jorio, et al. mapped the chiral structure of SWeNT® SG65 (Product No. 704148) using a continuously variable laser to excite the SWNTs.9
There are two supplementary bands observed in the Raman spectrum of SWNTs: the D band at ~ 1350 cm-1 signifies multiwall tubes, disordered carbon and microcrystalline graphite and the G band at 1500 to 1586 cm-1 is a consequence of the tangential stretching mode from graphitic-like materials. As a measure of the purity of SWNTs, the ratio of the height of the G band to that of the D band has been widely used.
However, care and attention must be paid when measuring this ratio due to the fact the G band is a resonant band and is therefore much more powerful than the D band. It is advised to assume that a high G:D ratio is a prerequisite condition for high purity SWNTs, but it is an inadequate assurance of purity since other techniques must be used synchronously with this parameter. For instance, other forms of graphitic carbon may provide a strong G band.
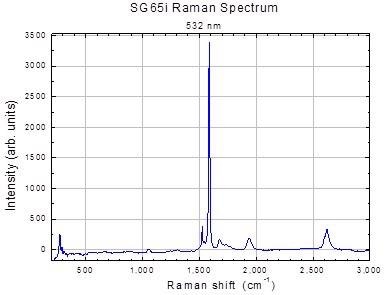
Figure 2. Ramen spectrum of SWeNT® SG65i SWNT (Product No. 773735). Image Credit: Merck Millipore Sigma
The Raman G:D ratio, with the cautions listed above can be used as a first measure of purity. A typical Raman Spectrum for SWeNT® SG65i (Product No. 773735) is shown in Figure 2.
Optical Absorption
Optical absorption (OA) measurements in the UV-Vis-NIR region illustrate peaks characteristic of single (n,m) species superimposed on the π-plasmon background. For instance, the (6,5) species absorb at 566 and 976 nm and in response fluoresce at 983 nm. 6-7 A (7, 6) absorbs at 645 and 1024 nm and fluoresces in response at 1030 nm. Therefore, as a basis for estimating the purity of SWNTs, these individual peaks have been used.10
Nair, et al. have developed a technique for computing the baseline for the spectrum, which then a calculates peak heights and areas for the individual (n,m) species.11 For simplicity, where the background becomes linear in the area of interest for SWNT characterization it is typical to transform the measured OA spectrum to the energy domain.
Figure 3 exhibits a standard OA spectrum for SWeNT® SG65i material. The inset highlights the spectrum in the more traditional form with the absorption marked as a function of wavelength. Measurements of the height of the strongest peak (P2B) and integration of the overall signal (S2B) can be utilized to guarantee consistency of the product. Predominant use of P2B as a control parameter for SWeNT® SG65i (Product Nos. 773735) and SG76 (704121) nanotubes where one specific tube type is dominant. P2B is described as the peak of the highest peak in the spectrum between 350 and 1,350 nm divided by the background at that wavelength.
P2B= Height of (6,5) or (7,6) Signal Peak
Height of Background Peak
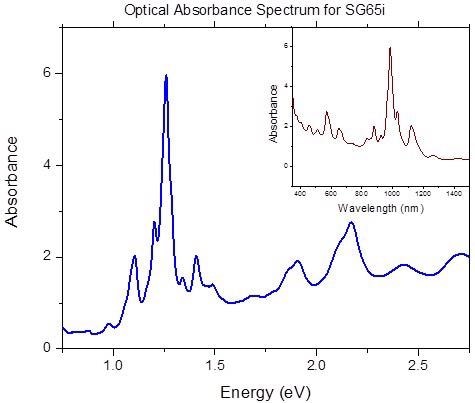
Figure 3. Optical absorbance spectrum in the region of UV-Vis-NIR of SWeNT® SG65i (Product No. 773735). Image Credit: Merck Millipore Sigma
Note, the OA technique as detailed here utilizes the OA spectrum measured after centrifuging and dispersing the SWNTs sample. It is used as a measure of chirality control as opposed to total purity. Measurement of the absorbance at a particular wavelength prior to and after centrifugation offers a measure of the dispersibility of the SWNTs.
Thermogravimetric Analysis (TGA)
A standard TGA curve for SG65i SWNTs is illustrated in Figure 4. TGA is utilized to evaluate the purity of the material. The principal quality parameters identified from the TGA analysis is the residual mass at 625 °C. This is a measure of the residual catalyst metals (now oxidized) kept in the material.
A subsequent peak in the derivative curve has been credited to the presence of other forms of graphitic carbon that oxidize at higher temperatures than SWNT. With advancements in both the catalyst manufacture and SWNT synthesis, additional forms of carbon have been limited to such a low level that they cannot be measured by TGA. The residual mass is revealed as a percentage normalized for the weight loss at 200 °C.
Residual Mass = Weight Loss at 625 °C
Initial Weight Loss
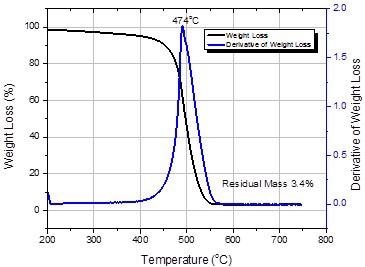
Figure 4. Thermogravimetric analysis of SWeNT® SG65i (Product No. 773735). Image Credit: Merck Millipore Sigma
Combining these three methods produces a good measure of purity and consistency with regards to the SWNTs. Yet, as continual development of SWNT applications takes place, functional testing, such as electrical conductivity measurements, will be necessary to link the purity data to SWNTs performance.
Applications of SWNTs
Extensive research came about due to the multitude of unique properties of SWNTs have to further develop its use in a wide range of applications.11 Their large surface areas and highly conductive nature are used to prepare conductive polymer composites and films, enhanced lithium ion batteries and supercapacitors. Optical properties enable their use as electrodes in solar cells, displays and groundbreaking solid-state lighting technologies. The semiconducting nature of some SWNTs species permits their adaptation to non-volatile memory elements, logic devices, sensors and security tags.
Most CNT manufacturing techniques generate a highly poly-disperse product – a variety of diameters and chiralities, compromising optical, electrical and chemical properties. Many SWNTs synthesis techniques generate a considerable amount of additional forms of metal, metal oxides and carbon which in most cases necessitate removal using costly secondary processing methods. Post-synthesis chiral separation methods are not at all economical and have low yield, starting with materials typically containing about 66% semiconducting species and 33% metallic species. Greater manufacturing costs and reduced capacity resulting from the above have asphyxiated market acceptance.
However, sudden developments by Southwest Nanotechnologies (SWeNT®) have addressed each of these problems with some degree of success due to the emergence of a new product dubbed SG65i (Product No. 773735). Representing an improvement over SG65 (Product No. 704148), the material considered to be the starting material for NIST’s (National Institute of Standards and Technology) Standard Reference Material. Until recently, SG65 was considered to be the most chirality-selective of available materials as synthesized. A side-by-side comparison of the two materials is illustrated in Table 1
Table 1. Comparison of SWeNT ® SG65i and SG65. Source: Merck Millipore Sigma
| Parameter |
SG65
(Product No. 704148) |
SG65i
(Product No. 773735) |
| (6,5) content (%SWNTs) |
< 40 |
> 40 |
| Semiconducting Tube Content (%) |
90 - 91 |
≥ 95 |
| Residual Mass (%) |
7.6 |
< 5 |
| Average Diameter (nm) |
0.8 |
0.78 |
| Ramen Q = (1-D/G) |
> 0.95 |
≥ 0.97 |
| Relative Purity (T1%) |
79 |
≥ 93 |
Both materials are produced using the patented CoMoCAT® synthesis process, well-known to be more selective for chirality and diameter rather than other methods. Considerable improvement of the catalyst system in SG65i has led to additional improvement of selectivity. These intensified properties are allowing rapid development of printed semiconductor devices, notably thin film transistors (TFTs).
TFTs demand a high concentration of semiconducting SWNT to accomplish the On/Off ratio and mobility necessary at reduced costs for general acceptance of OLED TV, for instance. SG65i will enable high yields of the required 99%+ semiconducting material, whereas alternative SWNTs that are commercially available usually only have the ‘natural’ proportion of semiconducting species, 67%. But beginning with a low initial concentration of semiconducting species and a wide distribution of diameters and chiralities, the yielded cost of such materials can be inhibiting for commercial applications. At ≥ 95% semiconducting SWNTs concentration, the task of purifying to the level required is greater simplified.
This high degree of flexibility and 'stretchability' and performance inherent to SWNT coatings will also allow printed, flexible semiconductors for flexible electronics, a variety of applications long pursued but not yet a commercial reality.
Another inspiring application area for SWNTs and specifically for SWeNT®, is in the medical arena, perhaps most notably in the treatment of cancers. Many research projects are underway that seem to be promising, depending on a high concentration of tubes of the (6,5) chirality, or other small diameter species. These projects benefit from the unique optical properties of tubes of this chirality – when irradiated with light in the near-infrared (NIR) region these SWNT fluoresce in the infrared, resulting in the capacity to heat internal tissue in the location of the cancer by irradiation of SWNT placed in the malignant tissue’s area. In this instance the high concentration of (6,5) SWNT in SG65i are expected to render the preparation of materials much more practical.
The adoption of SWNT replacements for ITO and conductive polymers such as PEDOT:PSS (Product Nos. 768642 and 739316) is becoming apparent for use in transparent conductive films (TCFs). Numerous applications of TCFs have optoelectronic properties exceeding the range of today’s SWNT-based coatings, but a variety of applications are well within the ‘window’ of capability for SWNTs. In these cases, a limiting factor was customer confidence in the availability of product that was suitably consistent, as well as a scalable manufacturing technique to assist emerging applications and a product that offers excellent optoelectronic performance.
To meet these requirements, SWeNT® has produced its most conductive grade CG300 (Product No. 775533), made by CoMoCAT®, recognized for its scalability and the consistent product it generates, with customized characteristics that make it particularly well-suited for including in the inks required for printing and coating.
Conclusion
Presently, commercial exploitation of the SWNT technology has been limited despite early enthusiasm regarding SWNTs materials and the exceptional amount of research inspired by their discovery. However, the pendulum is now swinging, moved by considerable progress made recently in multiple key areas. Therefore, it is expected that a dramatic expansion of applications will be seen as the hurdles are now being overcome.
Listed below are CoMoCAT® high purity SWNTs manufactured by SouthWest NanoTechnologies (SWeNT®), Inc. and available in research quantities exclusively from Merck.
Trademarks
SWeNT® and CoMoCAT® are registered trademarks of Southwest Nanotechnologies, Inc.
References and Further Reading
- Iijima, S. Nature, 1991, 354, 56.
- Arnold, M.S., Green, A.A., Hulvat, J.F., Stupp, S.I., Hersham, M.C. Nature Nanotechnology, 2006, 1, 60.
- Tan, Y., Resasco, D.E., J. Phys. Chem. B, 2005,109,14454.
- Meo, M., Rossi, M., Composite Science and Technology, 2006,66,1597.
- Tans, S.J., Devoret, H., Thess, A., Smalley, R.E., Geerligs, L.J., Dekker, C., Nature, 1997, 386,474.
- Bachilo, S.M., Strano, M.S., Kitrell, C., Hauge, R.H., Smalley, R.E., Weisman, R.B., Science, 2002,298,2361.
- Lolli, G., Zhang, L., Balzano, L., Sakulchaicharoen, N., Tan, Y., Resasco, D.E., J. Phys. Chem. B, 2006,110,2108.
- Miyata, Y., Yanagi, K., Maniwa, Y., Tanaka, T., Kataura, H., J. Phys. Chem. C, 2008,112,15997.
- Jorio, A., Santos, A.P., Ribeiro, H.B., Fantini, C., Souza, M., Viera, P.M., Furtado, C.A., Jiang, J., Balzano, L., Resasco, D.E., Pimenta, M.A., Phys. Rev. B,2005,72,075207.
- Itkis, M.E., Perea, D.E., Jung, R., Niyogi, S., Haddon, R.C., J. Amer. Chem. Soc., 2005,127,3439.
- Nair, N., Usrey, M., Kim, W-J., Braatz, R.D. Strano, M.S,. 2006, Anal. Chem., 2006,78, 7589.

This information has been sourced, reviewed and adapted from materials provided by Merck.
For more information on this source, please visit Merck.