Soil contains an array of micronutrients and these form the building blocks for crops destined to be eaten, processed, or fed to livestock. It is vital for both consumers and industry that micronutrients in soil be measured in order to track or prevent soil depletion while ensuring effective crop rotation and fertilizing.
Accurately analyzing soil for elemental content poses a number of challenges during sample preparation.
Conventional sample preparation involves an open vessel technique with an acidified sample being heated to near boiling before elements are extracted for analysis. This extraction method is slow, generally taking over 4 hours to complete. It is also prone to the loss of volatile analytes and contamination from environmental sources.
One alternative approach is to employ a closed vessel microwave digestion system - a rapid digestion method (<50 minutes) able to facilitate a complete sample digestion for analysis of total elemental content as opposed to extractable concentrations.
Use of a closed vessel technique will reduce the risk of environmental contamination while retaining the volatile analytes, which could be lost throughout open vessel sample preparation.
Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES) is commonly the preferred technique because of its multi-element and detection capabilities. Other techniques may be utilized (AA, ICP-MS), but ICP-OES offers a balance between cost-effectiveness, ease-of-use and speed of analysis.
This article explores the use of microwave assisted digestion and ICP-OES in the analysis of micronutrients within soil samples.
Sample Preparation
Following sample digestion (Table 1), the PerkinElmer Titan Microwave Preparation System (MPS) (Figure 1) was used to analyze soil samples from a range of sources, including garden plots, residential backyards, commercial farms and pastures.

Figure 1. PerkinElmer Titan MPS. Image Credit: PerkinElmer
Table 1. PerkinElmer Titan MPS Digestion Method using HCl and HNO3. Source: PerkinElmer
| Step |
Temp (°C) |
Pressure
Limit (bar) |
Ramp
Time (min) |
Hold
Time (min) |
Power
Limit (%) |
| 1 |
150 |
35 |
5 |
5 |
80 |
| 2 |
195 |
35 |
2 |
20 |
100 |
| 3 |
50 |
35 |
1 |
15 |
0 |
Sample preparation was done using an extraction method because this is generally used for soil samples. Two certified reference materials (CRMs) were analyzed directly and without dilution in order to confirm the methodology’s accuracy.
These were: Soil Solution A and Soil Solution B (High Purity Standards™, Charleston, South Carolina, USA).
Instrument Conditions and Method Parameters
A PerkinElmer Avio 200 ICP-OES was used to perform analyses. Table 2 shows the instrument conditions used, while Table 3 shows the method parameters in place.
Every solution had a final acid concentration of approximately 10% of the acid blend in order to match the digested samples’ relatively high acid concentration.
Table 2. PerkinElmer Avio 200 Instrument Conditions. Source: PerkinElmer
| Parameter |
Value |
| Nebulizer |
Meinhard® Glass Type K1 |
| Spray Chamber |
Glass Cyclonic Baffled |
| Sample Uptake Rate |
0.80 mL/min |
| RF Power |
1500 Watts |
| Nebulizer Gas |
0.70 L/min |
| Auxiliary Gas |
0.2 L/min |
| Plasma Gas |
8 L/min |
Table 3. PerkinElmer Avio 200 Method Parameters. Source: PerkinElmer
| Element |
Wavelength (nm) |
Plasma View |
Calibration Range (mg/L) |
| Al |
308.215 |
Radial |
25 – 500 |
| Ba |
233.527 |
Axial |
1 – 25 |
| Ca |
317.993 |
Radial |
25 – 500 |
| Co |
228.616 |
Axial |
1 – 25 |
| Cu |
327.393 |
Axial |
1 – 25 |
| Fe |
238.204 |
Radial |
25 – 500 |
| K |
766.490 |
Radial |
25 – 500 |
| Mg |
285.213 |
Radial |
25 – 500 |
| Mn |
257.610 |
Radial |
1 – 25 |
| Na |
589.592 |
Radial |
10 – 100 |
| Ni |
231.604 |
Axial |
1 – 25 |
| P |
178.221 |
Axial |
10 – 100 |
| S |
181.975 |
Axial |
10 – 100 |
| V |
292.464 |
Axial |
1 – 25 |
| Zn |
206.200 |
Axial |
1 – 25 |
| Y (int.std) |
371.029 |
Radial |
N/A |
| Y (int.std) |
371.029 |
Axial |
N/A |
Results and Discussion
Analysis was performed using a standard 2-point background correction. No other spectral correction formulas were used. A 3–point calibration was analyzed with concentrations ranging from 1 mg/L to 500 mg/L.
These concentrations were analyte dependent (Table 3). Correlation coefficients for all analytes were >0.999, clearly indicating the method’s accuracy and precision.
An Independent Calibration Verification (ICV) was analyzed and recovered within 10% of the true value for all analytes. This successfully confirmed the validity of the accuracy and calibration in terms of standard preparation.
Two CRMs were run to further validate method accuracy, and all analyte recoveries were found to be within 15% of the true value for both reference materials.
Once the analytical method had been successfully validated, the Titan MPS was used to digest collected soil samples.
The closed vessel technique significantly increases laboratory productivity, efficiency, and throughput, particularly when compared to the traditional open-vessel digestion process, which can take more than 4x longer.
Results from the soil sample analysis (Figure 2) revealed that some soils differed as much as a factor of 10x in concentration for specific elements but were otherwise consistent. This was not entirely unexpected because the samples were taken from a small geographical area.
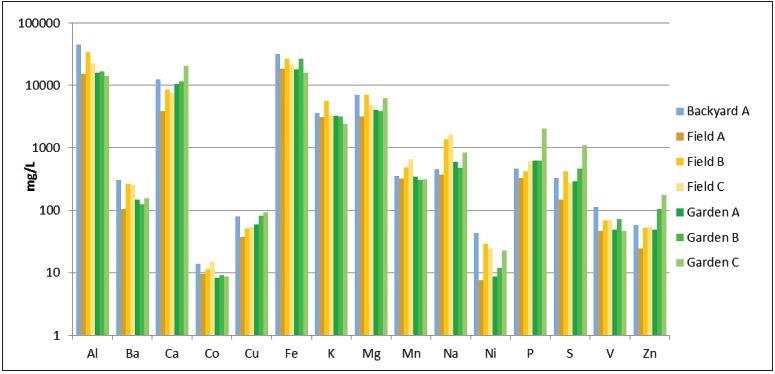
Figure 2. Results from analyses of soil samples (logarithmic scale). Image Credit: PerkinElmer
To assess any residual matrix effects, samples were fortified before digestion with analyte concentrations representative of what was anticipated in the samples. Spike recoveries were all found to be within 10% of the true value. This further confirmed accuracy of both the preparation and analysis methods (Figure 3).
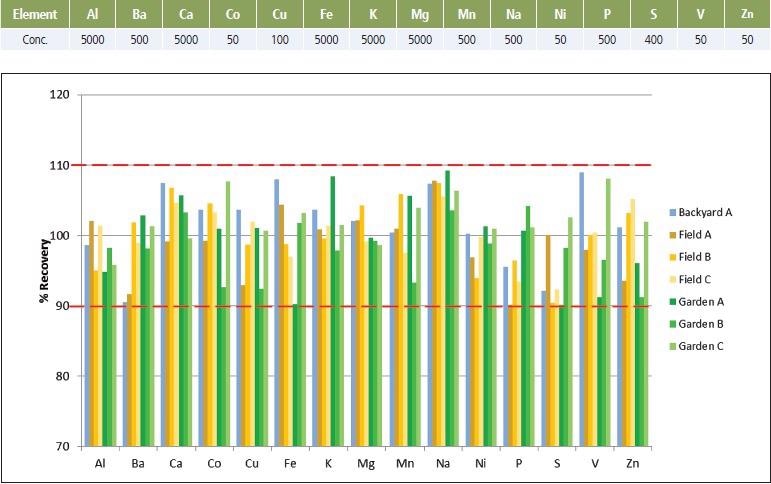
Figure 3. Spike concentrations and % Recoveries in soil samples. Image Credit: PerkinElmer
Conclusion
Employing the Avio 200 ICP-OES and the Titan MPS (Figure 4) facilitated the accurate measurement of micronutrients in a range of soil samples. The methodology developed and used for this study can easily accommodate an array of sample types, offering a more rapid and complete digestion than traditional open vessel methods.

Figure 4. PerkinElmer Avio 200 ICP. Image Credit: PerkinElmer
Effective sample digestion with the Titan MPS meant that per-sample matrix matching or the use of method of standard additions (MSA) were not required.
The combination of analytical results and spike recoveries for the samples, and the confirmation of method accuracy via CRMs, confirms that the use of a Titan MPS digestion system for sample preparation and an Avio 200 ICP-OES for final sample analysis offers an efficient method for the preparation and analysis of soil samples.
The Avio 200’s dual view capability and sizeable dynamic range meant that there was no need to perform multiple dilutions for elements at low and high concentrations.
This feature significantly increases laboratory productivity and sample throughput because all analyte concentrations can be determined during a single analysis.
Summary
Accurately detecting micronutrients in soils is essential in ensuring that environmental conditions are suitable for their intended use.
Knowledge of soil’s elemental content helps to avoid the use of contaminated soil, determine optimal fertilization and ideal crop rotation, all of which can help reduce overall cost. Identifying elemental components also allows time and resource savings through targeted treatment based on real-time analytical data.
A closed vessel microwave digestion procedure offers a rapid, effective means of meeting the challenges associated with variable soil types.
Utilizing this robust digestion procedure alongside the sizeable dynamic range and multi-element capabilities of an ICP-OES enables laboratories to increase sample throughput and overall operational efficiency.
Straightforward, fast and accurate sample preparation and analysis methods help safeguard proper plant nutrition. These are also essential in promoting efficient water usage and growth, maximizing production quality and quantity while minimizing environmental impact.
Acknowledgments
Produced from materials originally authored by Andrea Palpini and Nick Spivey from PerkinElmer, Inc.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.