Sponsored by ProtoFeb 2 2021
Some of the methods commonly used to track climate change and ocean acidification have significant disadvantages despite the phenomena being subjects of study for decades.
For example, scientists are at the mercy of computational models when measuring the acidity of large portions of the ocean instead of just small water samples; these models can contain their own variables and assumptions.

Image Credit: Jacki Moonves
Seawater has an average pH of 8.2, meaning it is normally close to neutral but slightly basic. However, the water can be made more acidic because of dissolved CO2 from the atmosphere.
Species dwindling or disappearing over time are among the devastating effects on marine life that can be caused by even a small decrease in the pH of the ocean. This means that not only is collecting accurate data from the whole ocean a massive challenge, it is also crucial.
Dr. Aaron Celestian of the Natural History Museum of Los Angeles County (NHMLAC), along with his colleagues at the University of Southern California and Caltech, is studying ocean acidity to obtain important data.
Celestian is NHMLAC’s curator of mineral sciences and specializes in mineral formation and interaction, specifically studying how they can inform climate change.
Dr. Celestian has been studying ocean animals to better understand the behavior of ocean minerals, specifically looking at animals whose shells are formed from the carbon-bearing minerals aragonite and calcite, different crystalline forms (polymorphs) of calcium carbonate.
Celestian and his team can gain a better understanding of fluctuations in seawater acidity by gaining more knowledge about the stability of minerals in these animals’ shells.
The carbon-bearing minerals used to form shells and corals are, according to Celestian, susceptible to acidic water at varying degrees.
For example, aragonite is more susceptible to low pH levels because it has nine oxygen atoms bound to calcium, making its bonds weaker than those of calcite. Conversely, calcite’s bonds are less likely to break because it has six oxygen atoms bound to calcium.
Shell structures can be slightly distorted, making them less stable, when magnesium is present in the water because it often substitutes for some of the calcium atoms in calcite.
In acidifying oceans, shells and other hard parts built from aragonite and Mg-calcite will be more readily dissolved and therefore more vulnerable.
Dr. Aaron Celestian, Curator of Mineral Sciences, Natural History Museum of Los Angeles County
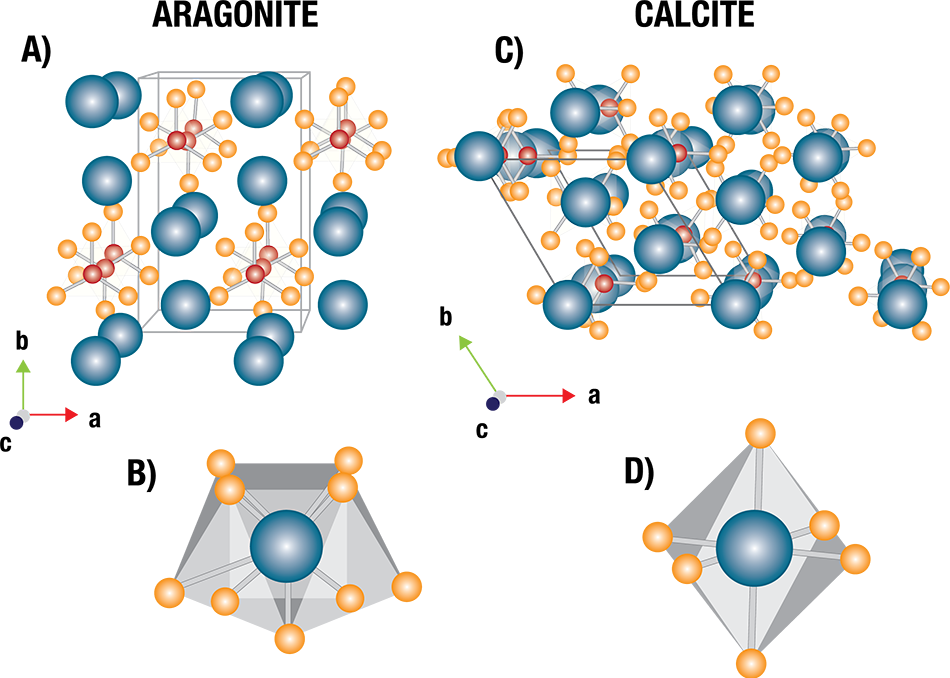
Structures of calcium carbonate polymorphs. Aragonite (left) has an orthorhombic crystal structure, while calcite (right) is trigonal. Image Credit: Proto
In order to investigate the susceptibility of these organisms to acidic water, special equipment is required.
Celestian and the C DisK-IV (Carbon Dissolution Kinetics) team have been merging x-ray diffraction (XRD) with marine mineralogy to more fully understand samples of ocean water obtained during several expeditions across the Pacific Ocean.
Their most recent voyage saw the group traversing from Hawaii to Alaska in the summer of 2017.
Previous research has measured dissolved carbon and ocean pH; however, this expedition additionally identified the concentration of carbonate minerals in seawater and measured the rates at which these minerals dissolve. Together, this work can provide valuable insight into the ways ocean acidification has changed over time.
The group collected sediments from the seafloor using a multi-corer during their voyages, also using seawater pumps to filter water from several different depths.
The scientists then used a Proto AXRD® powder diffractometer to analyze the contents of the filter samples. Powder XRD analysis is ideal for identifying crystalline structures, meaning percentages of calcium carbonate polymorphs could be determined at various ocean depths along the path.

A Proto AXRD Benchtop powder diffractometer. Image Credit: Proto
The group identified the ratio of aragonite to calcite in the water along their voyage by using XRD. Stable aragonite was found at a greater depth in the water samples taken near Hawaii; conversely, aragonite stability was observed at shallower depths as they moved toward Alaska. They concluded that the Alaskan waters were more acidic because the aragonite was not stable below a depth of 200 meters.
The more CO2 in the atmosphere, the more carbonic acid is produced, and the lower the pH of the oceans.
Dr. Aaron Celestian, Curator of Mineral Sciences, Natural History Museum of Los Angeles County
Animals that form shells will have difficulty maintaining their structure when the pH of the oceans gets lower, forcing them to expend more energy on forming and repairing their shells. The additional energetic demands may cause the animals to lose their ability to reproduce and survive, resulting in a population decline.
XRD is a helpful tool in the ongoing investigation of how mineral behavior informs ocean acidity.
Image Credit: Jacki Moonves

This information has been sourced, reviewed and adapted from materials provided by Proto.
For more information on this source, please visit Proto.