To reduce carbon dioxide (CO2) emissions, replacing traditional fuel-powered vehicles with battery-powered options is crucial. This greenhouse gas results from the combustion of fossil fuels, and limiting its input into the atmosphere will also affect global warming.

Image Credit: Metrohm Middle East FZC
Therefore, battery research focuses on discovering new materials with higher power density and energy, in addition to more efficient energy storage.
To develop viable new batteries, a number of critical parameters have to be established. Below, a few of the analytical parameters which can be established by utilizing high precision analytical instruments from Metrohm are outlined.

Image Credit: Metrohm Middle East FZC
What is in a Lithium Battery?
Lithium-ion batteries are the most common rechargeable batteries available on the market today. A battery is made up of a cathode (positive pole) and an anode (negative pole). An electrolyte facilitates charge transfer in the form of lithium ions between these two poles.
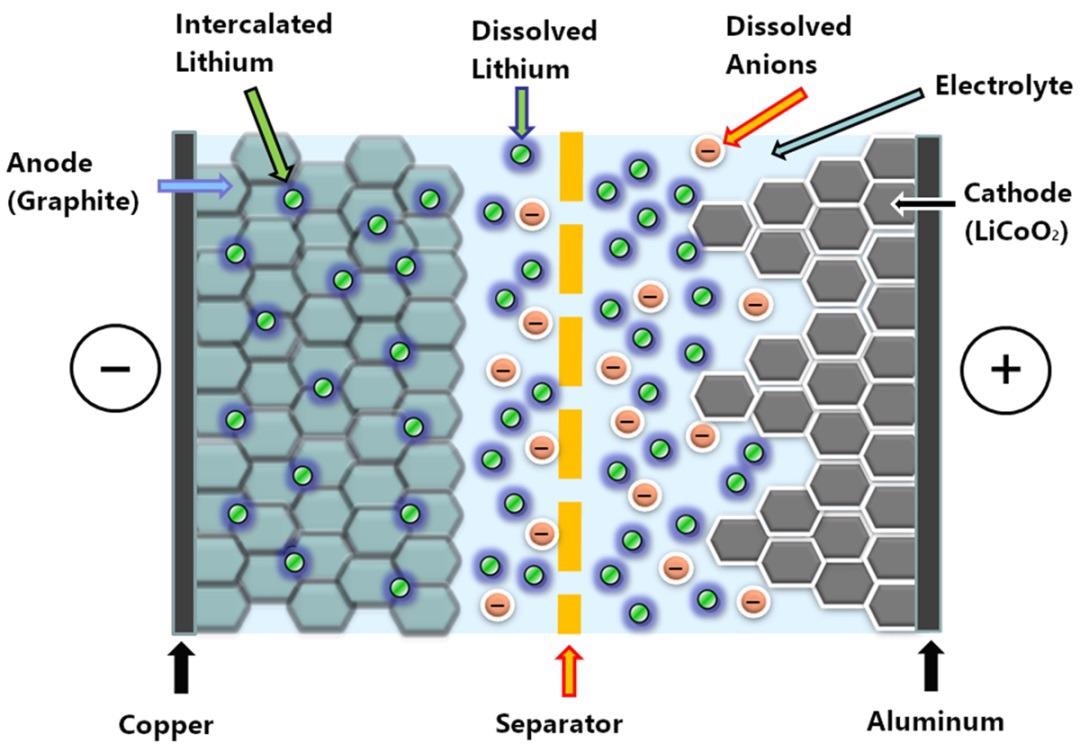
Figure 1. Cross-section illustration of a lithium ion battery. While the battery is being charged, lithium ions migrate from the cathode to the anode (from right to left), and during discharging they move from the anode to the cathode (from left to right). Image Credit: Metrohm Middle East FZC
As seen in Figure 1, a separator placed between anode and cathode prevents short-circuits. The cathode consists of metal oxides dotted with lithium ions applied to an aluminum foil, while the anode is created from graphite containing intercalated lithium applied to a copper foil.
The most common transition metals utilized in cathode materials are nickel, cobalt, manganese or iron. To facilitate charge transfer, the electrolyte is an anhydrous aprotic solvent containing a lithium salt (e.g., lithium hexafluorophosphate).
The separator is created from a porous material, acting as an insulator to stop short-circuits. The composition of all of these components has a huge effect on the battery characteristics.
Water Content in Battery Raw Materials
As water reacts with the conducting salt (e.g., LiPF6) to form toxic hydrofluoric acid, lithium-ion batteries should be free of water (concentration of H2O below 20 mg/kg). The ideal technique for establishing water content at trace levels is sensitive coulometric Karl Fischer titration.

Image Credit: Metrohm Middle East FZC
Water determination for solids is performed by utilizing the Karl Fischer oven technique – the residual moisture in the sample is evaporated and transferred to the titration cell where it is subsequently titrated.

Image Credit: Metrohm Middle East FZC
Transition Metal Composition of Cathode Materials
The cathode of a lithium-ion battery is typically created from metal oxides derived from manganese, cobalt, iron, nickel or aluminum. Solutions that contain the desired metal salts are utilized to produce the cathode.
The exact content of the metals present in the solution must be known for an optimized production process. Furthermore, the metal composition within the obtained cathode material should be established. Potentiometric titration is a suitable method to establish the metal content in starting solutions and the finished cathode materials.

Image Credit: Metrohm Middle East FZC
The following mixtures of metals or metal oxides can be analyzed potentiometrically:
- Cobalt, nickel and manganese in solutions
- Cobalt, nickel and manganese in cathode materials like cobalt tetraoxide (Co3O4), lithium cobaltite or lithium manganite
Potentiometric titration is also ideally suited for determining the purity of lithium salts. For lithium carbonate and lithium hydroxide, the purity is established by utilizing an aqueous acid-base titration. Using this technique, it is also possible to determine carbonate impurity within lithium hydroxide.

Image Credit: Metrohm Middle East FZC
For the assay of lithium nitrate and lithium chloride, the lithium is directly titrated by utilizing the precipitation reaction between lithium and fluoride in ethanolic solutions. The knowledge of other cations which might be present in lithium salts (and their concentration) is also of interest.
Ion chromatography (IC) can be used to determine various cations (e.g., sodium, calcium or ammonium).
IC is a precise and efficient multi-parameter technique used to quantify anions and cations over a large concentration range. The chromatogram in Figure 2 displays the separation of sodium, lithium and calcium in a lithium ore processing stream.
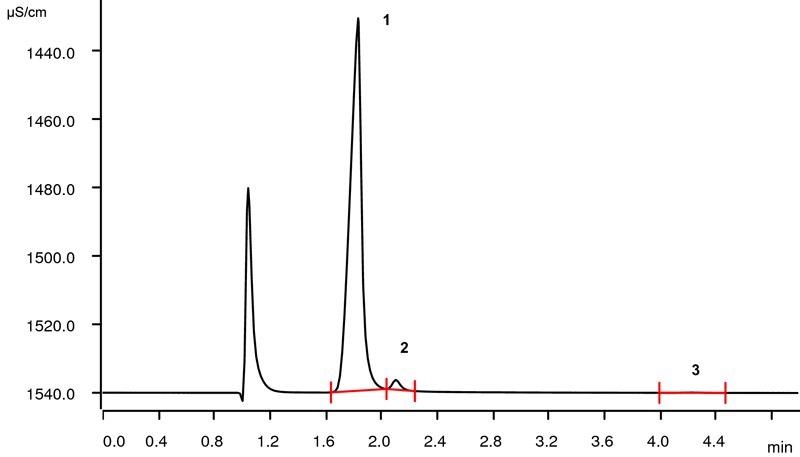
Figure 2. Ion chromatogram of the lithium ore processing stream (1: lithium, 23.8 g/L; 2: sodium, 1.55 g/L; 3: calcium, 0.08 g/L). Image Credit: Metrohm Middle East FZC
Eluated Ions and Decomposition Products
In the development and optimization of lithium-ion batteries, one item of special interest is the content of ions (e.g., lithium, fluoride and hexafluorophosphate) in the electrolyte or in eluates of different components.
Ion chromatography enables the determination of decomposition products in the electrolyte, or anions and cations eluted, for example, from finished batteries.
Furthermore, the Metrohm Inline Sample Preparation techniques can be automated and used for any sample preparation steps which might be needed (e.g., preconcentration, dilution, filtration).

Image Credit: Metrohm Middle East FZC
Since the rudimentary voltaic pile was developed over two centuries ago, battery technology has come a long way. The breakthrough innovation of the lithium-ion battery and its subsequent improvements has increased the utilization and accessibility of electronics, especially in the consumer market.
Electronics are more affordable, portable and are becoming more sustainable due to rechargeable or secondary batteries. Another reason that energy storage research, especially batteries, is a hot topic is due to the expansion of application possibilities.
For instance, only a decade ago, drones were the domain of the military industrial complex, and now a drone with a camera is a standard part of almost any successful photographer or influencer’s kit.
A drone now has an affordable price tag for a larger portion of the civilian population due to more cost-efficient materials and improved battery life. This kind of disruption is also happening in larger, more profitable markets.

Image Credit: Metrohm Middle East FZC
Due to their technological innovations and public relations, Tesla, still has a small, albeit growing, market share of the overall automotive market. Their success has challenged other established brands to recognize that a change from conventional combustion engines can be lucrative.
Ford and Volvo and are committed to being fully electric by 2030.1 General Motors (GM) has committed to not only be electric by 2035 but also for their business to be carbon neutral by 2040.2

Image Credit: Metrohm Middle East FZC
The automotive market is a high profile example of one industry that will drastically retool their sector, from manufacturing to sales, and this will happen over many other industries as there is a bigger focus on renewable energy sources and climate change from both consumers and governments.
To make these transformations possible, accurate and scalable R&D will be needed, and the hunt for better energy storage solutions is key to these changes.
Electrochemistry was the key to the discovery of energy storage and is the natural method of choice for future innovations.
Electrochemical Characterization Techniques for Lithium-Ion Batteries
This section highlights methods that will enable the characterization of various attributes of the electrochemical behavior of Li-ion batteries by using a high precision potentiostat/galvanostat.
In some instances, the difference between methods is due to carrying out the experiment in a different mode (i.e., galvanostatic or potentiostatic), and the additional information collected supplies a more complete picture of battery behavior.
Galvanostatic Intermittent Titration Technique (GITT)
The Galvanostatic Intermittent Titration Technique (GITT) is one of the first methods available to researchers to explore the properties of battery electrode materials. Typically performed on a half-cell, this method is a series of current perturbations followed by a relaxation time.
This supplies information regarding the electrode materials and thermodynamic properties, including the critical diffusion coefficient. All of this information supplies a better understanding of the electrochemical behavior to be expected by the materials.
Potentiostatic Intermittent Titration Technique (PITT)
Potentiostatic Intermittent Titration Technique (PITT) is similar to the GITT method, but the PGSTAT is operated in potentiostatic mode. A series of potential step perturbations is applied to the system, and current is measured as a function of time.
Both GITT and PITT are capable of determining the diffusion coefficient accurately.
When utilizing a PGSTAT in galvanostatic mode, the operator can also characterize the performance of Li-ion batteries by utilizing different current rates and charging and discharging during various cycles, known colloquially as ‘cycling’.
Using this method, researchers are able to understand the rate performance of the Li-ion battery, its capacity and the associated power and energy density. In battery research, this is the most commonly utilized method.
To ensure that a battery is fully charged while avoiding any battery overcharge, a constant current constant voltage (CCCV) procedure is typically applied. CCCV is the industry standard for Li-ion battery charging, and for this measurement, PGSTAT operates in both potentiostatic and galvanostatic modes.
Galvanostatic cycling is carried out within a safe potential window at which the electrolyte is stable. Any slight deviation from the potential cutoff may lead to poor cycle life.
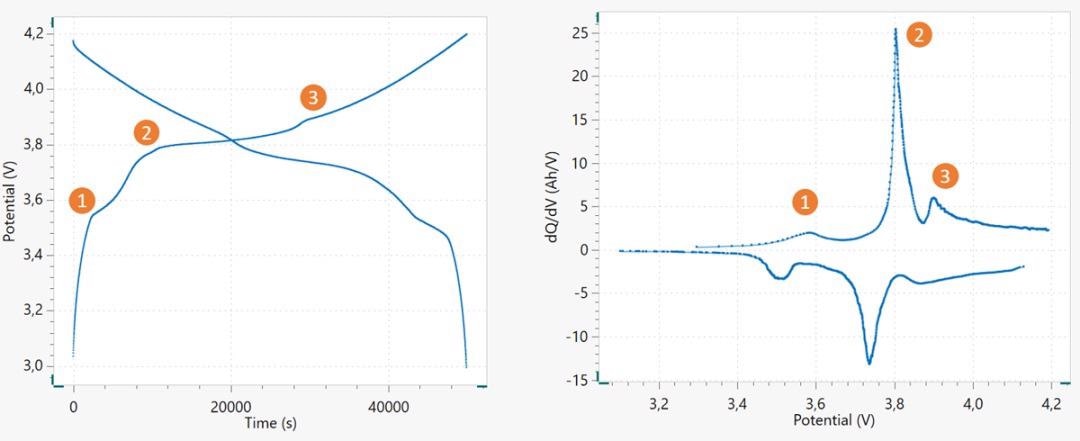
Voltage profile of a 18650 Li-ion battery, cycled at ~ C/15 (left), and its corresponding dQ/dV versus V plot (right). The corresponding peaks and plateaus are marked in the figures. Image Credit: Metrohm Middle East FZC
Acknowledgments
Metrohm Middle East FZC (MME) based out of Sharjah, UAE is the Regional Support Centre for Metrohm AG (Switzerland) which is responsible for sales, service and calibration of lab & process analytical instruments from the following countries –
UAE, KSA, Kuwait, Bahrain, Oman, Qatar, Egypt, Jordan, Lebanon, Iraq, Bangladesh, Pakistan, Sri Lanka, Ethiopia, Ghana, Sudan, Syria, Yemen, Somalia, Iran, Cyprus, Malta, Eritrea, Djibouti & Afghanistan.
Produced from materials originally authored by Dr. Reza Fathi from Metrohm International.

This information has been sourced, reviewed and adapted from materials provided by Metrohm Middle East FZC.
For more information on this source, please visit Metrohm Middle East FZC.