100% starting materials identification testing is a requirement according to the US FDA’s directive 211.84 for FDA-regulated industries such as Vaccines, Tobacco, Pharmaceutical, Food, Cosmetics, Animal veterinary products, etc.
STRam®-1064 is a Raman analyzer well-suited for this exact purpose. Utilizing patented STRaman® technology, it has the capacity to measure samples through dense packaging materials, including plastics, multilayer kraft paper sacks and HDPE containers.
The STRam®-1064 also possesses a long wavelength laser to suppress fluorescence.
The ID algorithm is able to isolate the sample signature by deducting that of the packaging material and cross-referencing it with library spectra for comparison to achieve identification.
The STRam® has a range of benefits, including the following:
- Rapid analysis that can be used anywhere: in labs, warehouses, processes, fields
- It prevents contamination as it is not necessary to open the material package for analysis
- Identification analysis using both commercial and user-created libraries
- Extensive range of applications that can be doen with the specialized STRaman technology, from temperature-sensitive samples to fluorescent samples
- FDA/21CFR Part 11 compliant software
In the work presented here, the STRam®-1064 was used for the identification/verification of raw materials tested in the regulated pharmaceutical warehouse environment as well as in the laboratory are demonstrated without opening the packages.
Samples
- Dextrose in a paper sack A (3 layers of brown kraft paper, 1 layer of PE film) (Figure 1)
- Dextrose in a paper sack B (1 layer of brown kraft paper, 1 layer of PE film) (Figure 2)
- Calcium chloride dihydrate in plastic bags (1 layer of blue PE, 1 layer of clear PE film). (Figure 3)
- Magnesium chloride hexahydrate in plastic bags (1 layer of blue PE, 1 layer of clear PE film).
- Sodium Lactate 60% USP in a thin HDPE (0.92 mm) and a thick HDPE (2.45 mm) container. (Figure 4)

Figure 1. Multilayered paper sack A for dextrose. Image Credit: B&W Tek

Figure 2. Dextrose in paper sack B. Image Credit: B&W Tek

Figure 3. Calcium chloride dihydrate in plastic bags. Image Credit: B&W Tek

Figure 4. Sodium lactate 60 in HDPE containers. Image Credit: B&W Tek
Method Development
In the laboratory, library spectra of pure samples were collected, which include dextrose, calcium chloride dihydrate, magnesium chloride hexahydrate, sodium lactate 60% and package materials.
The development of operation methods was carried out, using the acquisition parameters: Laser power 100%, integration time range from 10 to 80 seconds per sample for solids samples, and 10 to 60 seconds for sodium lactate 60% sample.
Sample Test
For dextrose, calcium chloride dihydrate and magnesium chloride hexahydrate, tests are performed in the raw material warehouse of a pharmaceutical company.
Tests for sodium lactate 60% were conducted in the B&W Tek HQ (Plainsboro, NJ, USA).
Sample preparation was not required for this analysis. Samples were scanned using the methods developed in the laboratory without opening the packing bags.
Results
Table 1. Test results for dextrose, calcium chloride dihydrate and magnesium chloride hexahydrate (solid). Source: B&W Tek
| Material |
Results |
| Dextrose in paper sack A |
Pass (HQI 80 ~ 88)
(Figure 5) |
| Calcium chloride dihydrate in plastic bags |
Pass (HQI 94 ~ 95)
(Figure 6) |
| Dextrose in paper sack B |
Pass (HQI 74) |
| Magnesium chloride hexahydrate in plastic bags |
Pass (HQI 90) |
Table 2. Test results for sodium lactate (liquid). Source: B&W Tek
| Material |
Results |
| Sodium lactate in a thick HDPE container |
Pass (HQI 80 ~ 88)
(Figure 7) |
| Sodium lactate 60 in a thin HDPE2 container |
Pass (HQI 99)* |
* Tests were done with a Focus Adaptor (RST-FA-xx) and Surface Regulator (RST-SR) attached.

Figure 5. Identification result of dextrose in paper sack A. Image Credit: B&W Tek
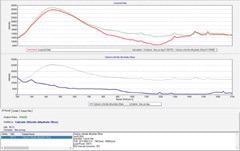
Figure 6. Identification result of calcium chloride dihydrate through plastic bags. Image Credit: B&W Tek

Figure 7. Identification result of sodium lactate 60% in thick HDPE2 container. Image Credit: B&W Tek
Conclusion
The STRam®-1064 was utilized in a pharmaceutical plant warehouse and in a laboratory to effectively verify raw material through unopened package materials.
This high-speed method is easy-to-use and can be performed without the need to extract any samples. The materials tested are identified with accuracy even through several layers of packaging materials such as blue, white and clear plastic bags and kraft paper.
Moreover, liquid samples were identified correctly through HDPE containers of varying thicknesses.

This information has been sourced, reviewed and adapted from materials provided by B&W Tek.
For more information on this source, please visit B&W Tek.