The WGS reaction (CO + H2O → CO2 + H2) represents one of the most critical processing steps in the industrial production of synthesis gas.
This process step is used to purify and adjust H2 composition in key downstream processes, for example, methanol synthesis (CO:H2 = 1:2), the Fischer-Tropsch synthesis of hydrocarbons (CO:H2 = n:(2n + 1)), and the Haber-Bosch process for the production of ammonia.
The development of robust, effective catalysts with excellent catalytic properties for the WGS reaction is, therefore, essential.
A number of promising options exist for high activity, lower temperature WGS systems, for example, the use of metals such as Au, Cu, or Pt used on reducible oxides such as CeO2 and titania-based nanocatalysts.
It has been shown that these metal/oxide catalysts demonstrate significantly higher activity than conventional Cu/ZnO WGS catalysts.
The design and optimization of ceria and titania-based WGS catalysts have thus far been impacted by disagreements and conflicting views around the nature of the active species/sites and the reaction mechanism.
One key challenge remains in terms of the characterization of the active state of the catalyst, the reaction mechanism, and the associated reaction intermediates, as this can be difficult to achieve at the elevated pressure and temperatures typically associated with WGS operation.
Recent studies into Pt-CeO2 nanocatalysts have shown promising WGS activity, however, with reactants and products able to be analyzed via a powerful mass spectrometer (Hiden QGA).
The catalysts’ structural features were characterized using a combination of multimodal characterization, enabling the specific features of the active metal-support interfacial bonding to be explored.
Most crucially, it was also possible to investigate how the temporal dynamic changes occurring during this process help enable high activity.
For the WGS reaction, complexities remain in terms of analyzing the dynamic characteristics of a Pt/CeO2 system at the atomic level. This is particularly the case when highlighting the synergistic effects of metal-support bonding at the perimeter region.
For example, the perimeter Pt0−O vacancy−Ce3+ sites formed in the active structure will be transformed at working temperatures. The appearance of these sites regulates the adsorbate behaviors, meaning that the dynamic nature of this site is a crucial mechanistic step for the WGS reaction.
To explore this further, the dynamics and heterogeneity of atoms in the active Pt species were investigated using a diverse array of techniques.
In situ TEM measurements were used to investigate the atoms within active Pt nanoparticles supported on ceria. This was done initially under CO and then under WGS.
It was noted that under CO, the Pt structure was barely discernible – most likely the result of the elevated temperature, rapid CO-induced mobility and the weakening of the Pt–Pt bonds from the strong repulsive interactions occurring between adsorbed CO molecules.
Pt atoms were found to remain dynamic under CO at 200 °C, most notable for those atoms deep in the nanocluster core and either at or close to the interface with oxide.
Under the WGS, however, practically all the outer Pt atoms became more stabilized/localized, other than those located at the cluster’s perimeter sites.
These observations indicate a degree of complexity within the nanocluster and that this changes dynamically and heterogeneously under in situ conditions.
A range of complementary probes were applied to highlight the changes to the Pt and ceria actives sites under reaction conditions. This was necessary in order to comprehensively characterize the structural evolution of the catalysts, heterogeneity and dynamic behavior of atoms located at various places of the nanostructure.
Both the structural features and the nature of these dynamic changes are linked to reactivity.
These findings will assist scientists in better understanding the working mechanism of this catalyst, empowering them to design future catalysts offering improved performance and lower costs. These techniques can also be applied to the study of other catalysts.
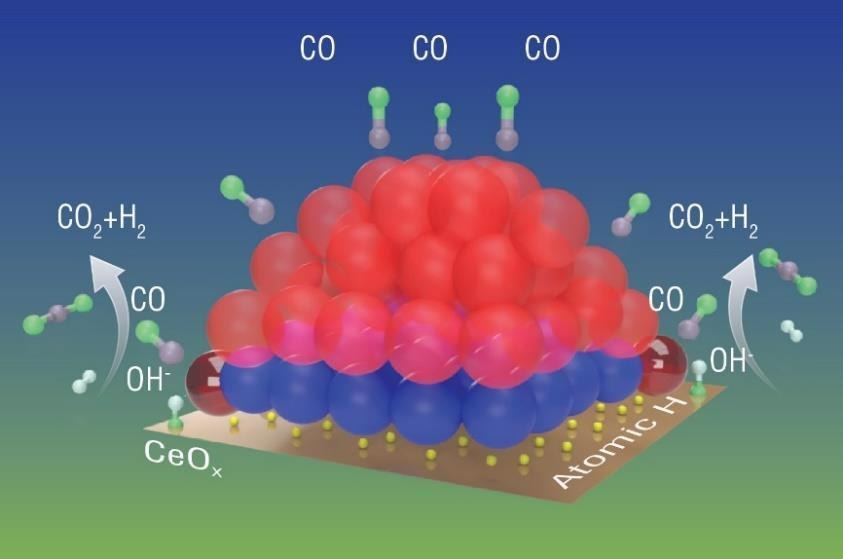
Image Credit: Hiden Analytical
The schematic presented here illustrates a working Pt/CeO2 catalyst operating under the WGS reaction.
The red and blue spheres represent metallic (zero state) Pt atoms and oxidized (2+) Pt atoms, respectively. Under the reaction condition, a number of platinum atoms around the nanoparticle’s periphery (shiny dark red) were activated and became involved in the reaction.
These activated platinum atoms will transfer oxygen from OH groups (initially from water molecules) to carbon monoxide (CO), transforming this to CO2 and leaving the H to combine with an atomic hydrogen to form H2.
Reference
“Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction” Nature Communications (2021) 12, 914 https://doi.org/10.1038/s41467-021-21132-4
Acknowledgments
Produced from materials originally authored by Yuanyuan Li, Department of Materials Science and Chemical Engineering, Stony Brook University.

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.