Fully hydrogenated graphene is often referred to as ‘graphane.’ This material is made up of 7.7 % hydrogen by mass, offering a range of distinct and highly useful physical, electrical and optical properties.
Pristine graphane is challenging to isolate in bulk form (much like graphene itself), but it is possible to chemically hydrogenate graphite and similar carbon allotropes in order to yield similar materials.
These materials - colloquially termed hydrogenated graphene - are stable under ambient conditions but will liberate hydrogen gas upon thermal decomposition above 400 °C. This property makes them promising candidates for hydrogen storage.
Studies are ongoing into the viability of hydrogenated graphene as a hydrogen storage solution,1,2 with fundamental studies looking into scaling up the material’s synthesis, characterization and testing capacity.
Accurate characterization and quantification of the generated gases is, therefore, key to identifying both the material’s volumetric and gravimetric hydrogen storage capacity and the purity of the evolved hydrogen gas.
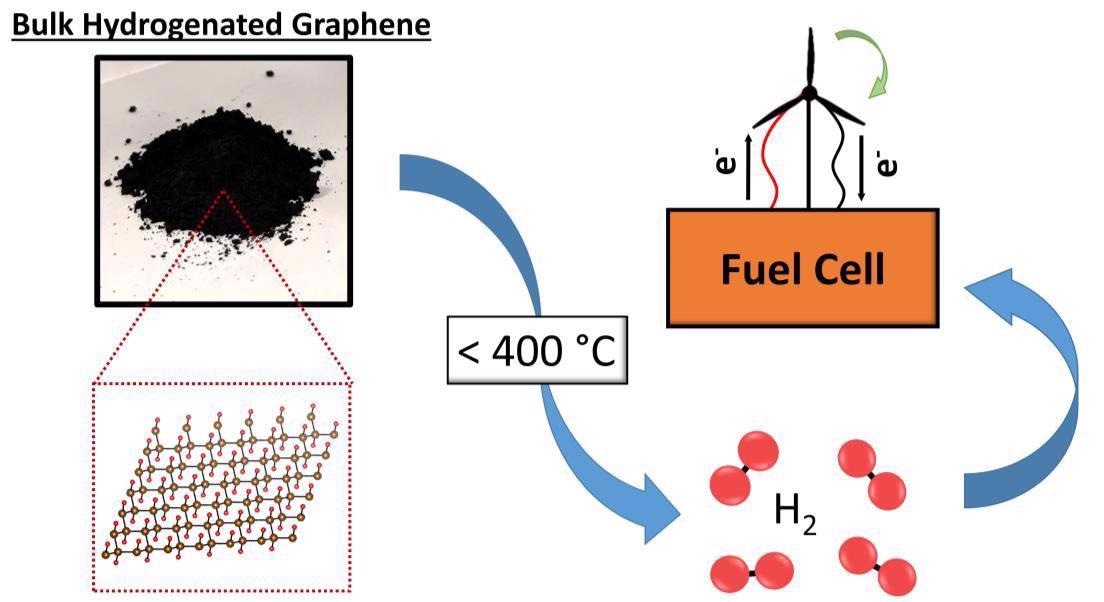
Image Credit: Hiden Analytical
Hydrogenated graphene was characterized via thermal gravimetric analysis coupled with mass spectrometry. This was done to help elucidate the dynamics of thermal decomposition.
A Hiden HPR-20 mass spectrometer was used to characterize evolved gases in real-time as samples of hydrogenated graphene were heated at a controlled rate.
The composition and quantity of gaseous species generated from the thermal decomposition of hydrogenated graphene samples were also identified via the decomposition of a known amount of hydrogenated graphene in a sealed pressure reactor.
This reactor was directly plumbed to the calibrated Hiden HPR-20 mass spectrometer.
It was noted that both water and ammonia were among the gaseous products observed during decomposition, alongside hydrogen gas itself.
The accurate differentiation of water and ammonia species can pose a challenge to mass spectrometry techniques due to the spectral overlap of the two species’ mass fragments.3
Appearance Potential Soft Ionisation (APSI) mode was employed to tune discrete ion energies for individual mass channels.
This made it possible to selectively identify the parent ions and minimize spectral overlap, resulting in the unambiguous characterization and quantification of all evolved gases (for example, water and ammonia species) throughout the decomposition of the hydrogenated graphene.
Hydrogenated graphene was evaluated as a means of storing and delivering hydrogen gas on-demand, thanks to this characterization and other complementary techniques.1,2
Hydrogenated graphene was found to possess a hydrogen storage capacity of ~3.2 wt.% using the developed synthetic protocol. This is approximately 42% of the theoretical value of graphane.
Ongoing improvements to these synthetic protocols are expected to increase the H2 storage capacity of this material, but the results already show that multi-gram quantities of hydrogenated graphene can be employed in the safe generation of practical quantities of H2 gas on demand.
References
- J.R. Morse, D.A. Zugell, B.R. Matis, H.D. Willauer, R.B. Balow, J.W. Baldwin, Int. J. Hydrogen Energy 45 (2019) 2135.
- J. Power Sources 494 (2021) 229734.
- “APSI mode simplifies MS spectral analysis” https://www.hidenanalytical.com/wp-content/uploads/2017/06/HAPR0174_APSI-mode-simplifies-MS-spectral-analysis.pdf (accessed Aug. 20, 2021).
Acknowledgments
Produced from materials originally authored by James R. Morse from the Naval Research Laboratory, Materials Science & Technology Division, USA

This information has been sourced, reviewed and adapted from materials provided by Hiden Analytical.
For more information on this source, please visit Hiden Analytical.