AZoM talked to Zewen Zhang, who worked in collaboration with Professor Yi Cui to image the solid-electrolyte interphase in lithium batteries. This novel imaging technique could pave the way for next-generation battery technology.
Please can you introduce yourself, your background, and how you came to be involved with this research?
I am Zewen Zhang, currently a Ph.D. student in the Materials Science and Engineering Department at Stanford University, US. I hold my Bachelor of Engineering degree from Tsinghua University, China. I have been working on batteries since my sophomore year. Here at Stanford, I am working in Prof. Yi Cui's lab and my research has mainly focused on developing methods to characterize the materials and interfaces in batteries in their native states to guide the structure and chemistry design and enable better batteries.
Why do lithium-ion batteries draw such a significant research focus currently?
The development of clean energy generation, transmission, and distribution technology is critical to progress toward a sustainable future. Rechargeable batteries, especially lithium-based batteries, are probably the most suitable technology to store renewable energy and help decarbonize the grid.
What is the solid-electrolyte interphase, or SEI, in batteries?
The solid-electrolyte interphase, or SEI, is the interphase formed on the electrode resulting from the sacrificial decomposition of electrolyte components. Specifically, almost all electrolytes in lithium-ion or lithium metal batteries are thermodynamically unstable against negative electrode working potential.
This inherent instability will result in the decomposition of electrolyte solvent as well as the lithium salt in the electrolyte. The decomposition product will form a layer on the electrode; this layer resembles the nature of solid-electrolyte and is therefore called the solid-electrolyte interphase (SEI).
How does this layer compromise the function of lithium-ion batteries?
To be fair, the formation of the SEI is not completely bad for batteries. As described earlier, the electrolyte is electrochemically unstable against negative electrode working potential. The existence of SEI actually helps make this thermodynamically unstable electrolyte-electrode interface metastable, and thus enables the stable cycling of batteries. However, the growth of this SEI will consume active lithium (or capacity), leading to capacity decay.
On one hand, the uncontrolled growth of the SEI will continuously consume active capacity. On the other hand, the build-up of SEI will increase the cell impedance over time and leads to a decrease in the round-trip energy efficiency of batteries.
Why was it so difficult to image this layer previously?
Before 2017, pictures of SEI remained elusive to the battery community. First, SEIs are just tens of nanometers thick. Only very few limited characterization tools have such a high spatial resolution. Transmission electron microscopy (TEM) is probably the only feasible choice to investigate these interphases.
Second, these battery materials and interphases are extremely air and moisture-sensitive. Even minimum exposure will alter their chemistry, nanostructure, etc. However, these contaminations are inevitable in conventional TEM sample preparation and transfer procedures.
Third, these battery materials and interphases are easily subject to high-energy electron beam damage. In the TEM column, electrons are accelerated to have 300 keV energy. Such a high-energy beam would easily destroy the sample. This can become even more problematic when we zoom in further because the beam intensity will be even higher.
Prof. Yi Cui's lab first adapted and developed cryogenic electron microscopy (cryo-EM) for battery materials, where the established cryo-transfer procedure helped prevent the contamination of air and moisture, and the low-temperature imaging conditions greatly improved the beam tolerance of the sensitive battery materials. We got the first-ever atomic resolution images of sensitive battery materials and interphases.
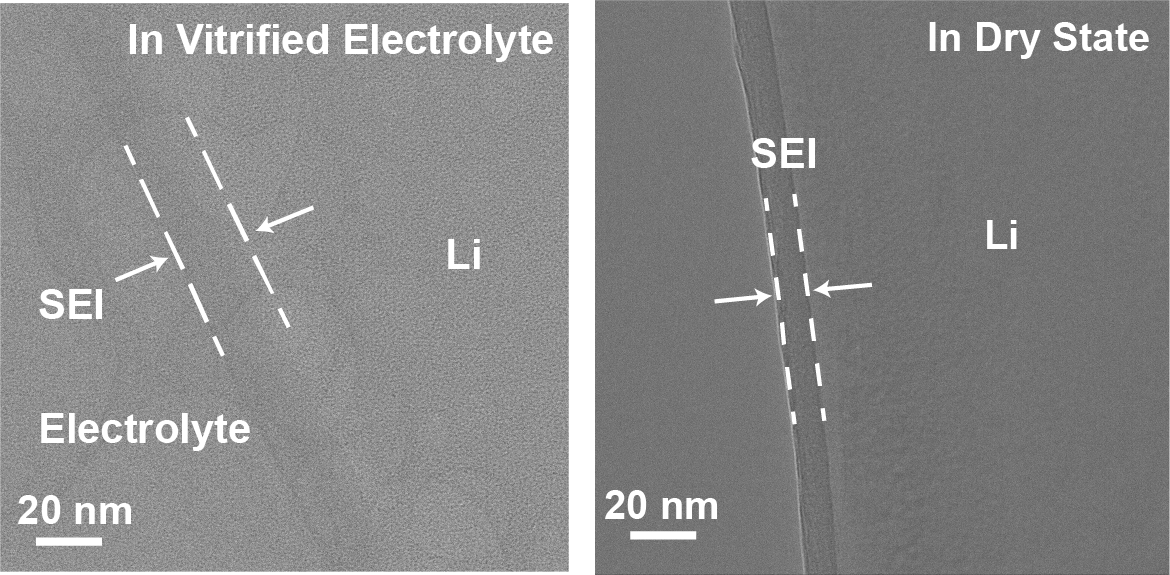
HRTEM of SEI on Li metal dendrite in vitrified electrolyte and in dry state. The thickness difference in both states indicates the swelling state in the SEI layer in their native liquid electrolyte environments.
Now, we are pushing this limit even further. In working batteries, the electrode materials are in a liquid electrolyte environment. In our first generation work, the liquid electrolyte is removed for sample preparation. Now, we would like to preserve the liquid electrolyte environment with the battery materials together to probe the interface in the native electrolyte environment. The challenge here is at least twofold.
On one hand, high-resolution TEM samples need to be at most a few hundred nm thick; obtaining such a thin sample of both liquid electrolyte and solid electrode materials is really challenging. On the other hand, the organic electrolyte is even more sensitive to the electron beam than the SEI, and therefore the imaging protocols should be carefully tuned. Therefore, it has remained difficult over the years.
How were you able to obtain a high-res image of the SEI? What methods were used?
For the sample preparation part, we adopted and developed the thin-film vitrification method to preserve the electrolyte environment, with lithium metal dendrite embedded in the vitrified electrolyte thin film. Here, vitrification means making something like glass. Specifically here, we are freezing the liquid electrolyte so fast that it does not crystalize and maintains approximately the native liquid environment-like local structure.
For the imaging part, we use the low dose technique combined with a direct electron detector to minimize the total electrons we use to form the high-resolution image.
Why was blotter paper necessary in this process?
The blotting procedure is critical; it removes extra liquid on the TEM grid so that we can obtain a thin sample that is appropriate for high-resolution TEM characterization.
What do these images tell us about the swelling of the SEI? How does this correlate to battery performance?
By comparing the thickness of dry state and wet state SEI, we firstly reported the swelling state of SEI in a liquid electrolyte environment. We also found that the swelling behavior is almost universal in different liquid electrolyte systems. By defining a swelling ratio, the thickness ratio of wet and dry state SEI, we discovered that the higher degrees of SEI swelling tend to exhibit poor electrochemical cycling.

Image Credit: Smile Fight/Shutterstock.com
Ultimately, why is the ability to image this layer significant? How can this inform attempts to control its negative effects?
SEIs are often regarded as “the most important but least understood component” in batteries. The rational design of the battery interface necessitates an atomic-level understanding of these key components. With the knowledge of local chemical and structural information in the SEI, we could establish the composition-structure-property relation for engineering principles.
How significant do you believe your research to be to improving battery charging rates?
Interfaces are critical components in determining battery performances. The detailed fundamental understanding of the SEI will help promote the rational design, and this will also be the case for fast charging applications.
What is personally the most exciting aspect of this new research to you?
The most exciting aspect of this new research is that we can leverage the power of interdisciplinary research to generate new knowledge. Specifically, we are now able to probe these otherwise inaccessible length scales in the electrochemical systems and provide new findings that are fundamentally different from what has been hypothesized before.
These otherwise inaccessible length-scale understandings of these critical components lead to a lot of valuable insights, either supporting previous hypotheses or generating new ones.
You have previously stated that you hope to be able to produce 3D images of the SEI. How would you begin to approach this?
We are working on this exciting new direction to utilize tomography techniques to resolve the interphases and materials in their native states in 3D. This will also be a combined effort with Stanford University and SLAC.
More from AZoM: Graphene Batteries in Electric Vehicles
Where can readers find more information?
Yi Cui Group Website:
http://web.stanford.edu/group/cui_group/
Link to this paper:
https://www.science.org/doi/10.1126/science.abi8703#:~:text
Link to Google Scholar:
Yi Cui: https://scholar.google.com/citations?user=lNmR2vAAAAAJ&hl=en
Zewen Zhang: https://scholar.google.com/citations?user=kVwAChsAAAAJ&hl=en
About Professor Yi Cui and Mr. Zewen Zhang
 Mr. Zewen Zhang is currently a 5th year PhD student at Stanford, working with Prof. Yi Cui and Prof. Wah Chiu. He received his B.E. degree in Materials Science and Engineering from Tsinghua University. He has been recognized with several honors and awards, including the Stanford Interdisciplinary Graduate Fellowship, the Min Zhu and Susan Xu Engineering Fellowship at Stanford.
Mr. Zewen Zhang is currently a 5th year PhD student at Stanford, working with Prof. Yi Cui and Prof. Wah Chiu. He received his B.E. degree in Materials Science and Engineering from Tsinghua University. He has been recognized with several honors and awards, including the Stanford Interdisciplinary Graduate Fellowship, the Min Zhu and Susan Xu Engineering Fellowship at Stanford.
Professor Yi Cui is the director of the Precourt Institute for Energy, co-director of the StorageX Initiative, Fortinet Founders Professor of Engineering in the Department of Materials Science and Engineering, and of photon science at SLAC National Accelerator Laboratory. He earned his bachelor’s degree in Chemistry in 1998 from the University of Science & Technology of China, and his Ph.D. in Chemistry from Harvard University in 2002. Cui was a Miller Postdoctoral Fellow at the University of California, Berkeley, from 2002 to 2005, before joining the Stanford faculty.
 As a preeminent researcher of nanotechnologies for better batteries and other sustainable technologies, Cui has published more than 500 studies and is one of the world’s most-cited scientists. He is an elected fellow of the American Association for the Advancement of Science, the Materials Research Society, the Electrochemical Society, and the Royal Society of Chemistry. He is an executive editor of Nano Letters and co-director of the Battery 500 Consortium.
As a preeminent researcher of nanotechnologies for better batteries and other sustainable technologies, Cui has published more than 500 studies and is one of the world’s most-cited scientists. He is an elected fellow of the American Association for the Advancement of Science, the Materials Research Society, the Electrochemical Society, and the Royal Society of Chemistry. He is an executive editor of Nano Letters and co-director of the Battery 500 Consortium.
He has founded five companies to commercialize technologies from his lab: Amprius Inc., 4C Air Inc., EEnotech Inc., EnerVenue Inc, and LifeLabs Design Inc. Cui’s honors include Global Energy Prize (2021), Department of Energy Lawrence Award (2021), Materials Research Society Medal (2020), and Blavatnik National Laureate (2017).
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.