Cryo-electron microscopy (cryo-EM) is increasingly being utilized as a mainstream technology to study the architecture of protein assemblies, viruses, and cells at molecular resolution. This article focuses on different aspects of cryo-EM, including its advantages and disadvantages, applications, the difference between cryo-EM and electron microscopy (EM) techniques, along with recent studies involving cryo-EM technology.

Scientist preparing samples for Cryo-electron microscopy in liquid ethane under liquid nitrogen temperature. Image Credit: PolakPhoto/Shutterstock.com
What is Cryo-EM?
Cryo-EM is referred to as a form of EM where a transmission electron microscope (TEM) is used to obtain the image of radiation-sensitive specimens under cryogenic conditions. The phrase cryo-EM is often used for several experimental methods, such as single-particle cryo-EM, electron crystallography, and cryo-electron tomography.
Every cryo-EM method can be used separately and in hybrid approaches, where information obtained from cryo-EM is merged with complementary information obtained from nuclear magnetic resonance (NMR) spectroscopic and X-ray crystallographic approaches. Currently, cryo-EMs have become more accessible and much easier to use, and are used for imaging biological specimens such as bacteria, plunge-frozen cells, and intact tissue sections.
Difference Between EM and Cryo-EM
Conventional EM methods use staining, dehydration, and chemical fixation of specimens to obtain their image. The interaction between the organic matter and electrons extensively damages the biological specimens in EM.
Meanwhile, cryo-EM preserves the native hydrated state of specimens, as this technology does not require additional methods for imaging specimens.
The electron irradiation breaks the chemical bonds within the specimens, which leads to the generation of free radicals that further cause secondary damage. Although low electron doses can preserve specimens by minimizing the radiation damage, such low doses lead to noisy images.
Cryo-EM can effectively resolve this issue, as imaging in this technique is performed using frozen specimens that are maintained at either liquid helium or liquid nitrogen temperatures, which reduces the adverse impacts of electron irradiation on the specimens.
The biological specimens are rapidly vitrified in a glass-like ice layer and then imaged at liquid helium and/or liquid nitrogen temperatures. At nitrogen temperature, radiation damage is substantially reduced, which allows the use of a higher electron dose to obtain images with a good signal-to-noise ratio. Both liquid nitrogen and helium can also be used to obtain three-dimensional (3D) reconstructions of specimens at a near-molecular level through the cryo-EM method.
Advantages and Disadvantages of Cryo-EM
The cryo-EM technique does not require crystals and is suitable for imaging proteins and their large molecular weight complexes, as well as the structural analysis of membrane proteins. Additionally, the technique preserves the functional state and native activity of biological samples and resolves the structures that were not resolved by other techniques, such as X-ray crystallography.
However, cryo-EM is ineffective for imaging very small proteins and requires a considerable amount of time to generate sample images. Additionally, the technique requires very high sample homogeneity, which increases the difficulties of obtaining high-resolution images of flexible proteins. Moreover, the current resolution of cryo-EM is not sufficient for pharmaceutical research and development, as the images obtained by this technique have a low signal-to-noise ratio in certain instances.
Major Applications of Cryo-EM Methods
Cryo-electron tomography is used to create 3D structures of macromolecular assemblies based on a series of images, with every image obtained at a different tilt relative to the incident electron beam direction. However, imaging of thicker specimens, such as eukaryotic cells or large viruses, is often performed using an imaging filter that enhances the contrast by removing inelastically scattered electrons.
These images are then combined computationally to generate tomograms, which are primarily the 3D images of the specimens. Several insights, such as the lateral arrangement of membrane protein assemblies and the organization of the bacterial cytoskeleton, emerged from these tomographic studies. Other applications of cryo-electron tomography include the investigation of different cytoskeletal and filamentous assemblies and imaging of thin mammalian cell regions.
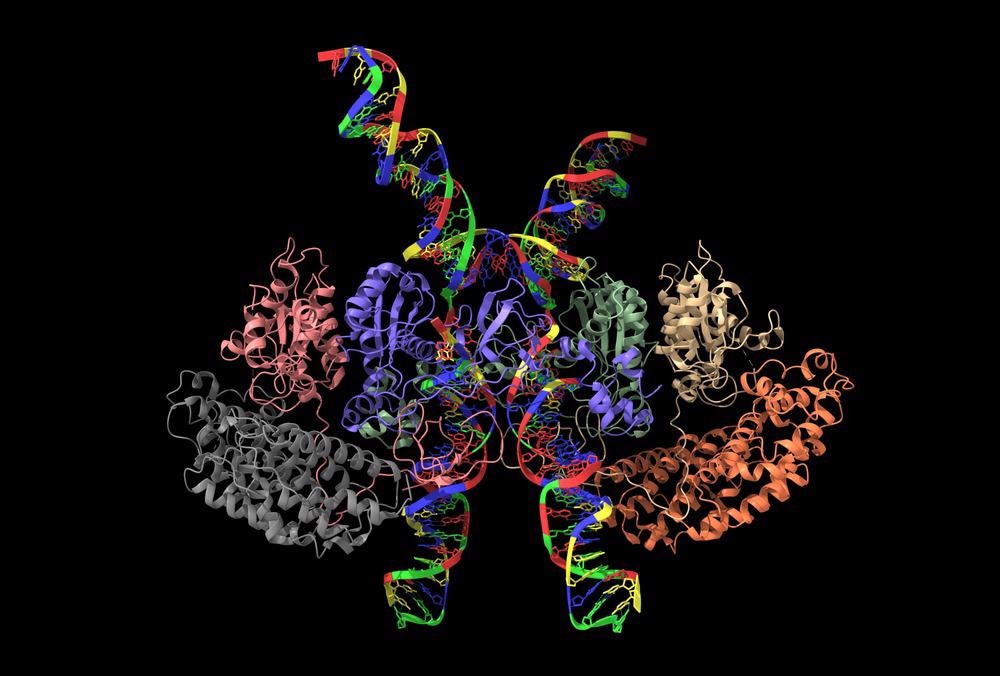
Cryo-EM structure of human T-cell leukemia virus type-1 (HTLV-1) intasome. Image Credit: Volodymyr Dvornyk/Shutterstock.com
The single-particle analysis is the most commonly used cryo-EM technique. In single-particle analysis, data obtained from several two-dimensional (2D) projection images that feature matching copies of a protein complex in various orientations are combined to produce a 3D reconstruction of a biological structure. The highest resolutions using the single-particle approach were achieved while imaging icosahedral viruses. Other applications of single-particle imaging include reconstructions at 10 Angstrom resolutions of dynamic protein complexes and analysis of conformationally heterogeneous specimens.
Recent Studies Using Cryo-EM
Recently, cryo-EMs were used extensively to collect the images of proteins and other molecular structures and store them in the Electron Microscopy Data Bank (EMDB), a popular repository for EM-solved structures. These structures are expected to be used to identify the working of proteins, and their role in disease diagnosis and treatment.
Cryo-EM was also used to identify the structural changes in coronavirus disease 2019 (COVID-19) variants to understand the evolution of the virus, which increased their infectivity. High-resolution cryo-EM was employed to detect the changes in the spike protein structure of beta and alpha variants. The research was published in the journal Nature Communications.
Additionally, cryo-EM was employed to identify the complex functions of cellular processes for the first time. Researchers, publishing in the journal Cell Discovery, used cryo-EM to closely observe the ATP13A2 protein present in brain cells. The protein leads to neurogenerative diseases such as Parkinson's disease when it loses functionality owing to a genetic mutation. The samples were frozen to temperatures between -238° Fahrenheit and -460° Fahrenheit during the imaging.
Conclusion
To summarize, cryo-EM has emerged as one of the effective imaging techniques to determine the structures of biological assemblies and macromolecules. In the future, cryo-EM can be potentially used for structure-based drug discovery. However, more research is required to use the technique for imaging membrane protein complexes and low-copy-number macromolecules in their native environment.
More from AZoM: Microchannel Plate Detectors in Electron Spectrometers
References and Further Reading
Subramaniam, S., Patwardhan, A., Pierson, J. et al. Cryo-electron microscopy: A primer for the non-microscopist. The FEBS Journal 2013. https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.12078
Benjin, X., Ling, L. Developments, applications, and prospects of cryoelectron microscopy. Protein Science 2020. https://onlinelibrary.wiley.com/doi/10.1002/pro.3805
Callaway, E. Revolutionary cryo-EM is taking over structural biology. Nature 2020. https://www.nature.com/articles/d41586-020-00341-9
Sweger, Z. Microscopy opens doors for studying neurological diseases. 2021. https://phys.org/news/2021-12-microscopy-doors-neurological-diseases.html
Wrobel, A.G., Benton, D.J., Roustan, C. et al. Evolution of the SARS-CoV-2 spike protein in the human host. Nat Commun 13, 1178 (2022). https://www.nature.com/articles/s41467-022-28768-w#citeas
Broadwith, P. (2021, October 8). Explainer: What is cryo-electron microscopy. Chemistry World. https://www.chemistryworld.com/news/explainer-what-is-cryo-electron-microscopy/3008091.article
What is Cryo-EM? (2019, January 7). Stanford-SLAC Cryo-Electron Microscopy. https://cryoem.slac.stanford.edu/what-is-cryo-em
Chen X, Zhou M, Zhang S, et al. Cryo-EM structures and transport mechanism of human P5B type ATPase ATP13A2. Cell Discov. 2021;7(1):106. Published 2021 Nov 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8564547/
Castón, J.R. Conventional electron microscopy, cryo-electron microscopy and cryo-electron tomography of viruses. Subcell Biochem 2013. https://pubmed.ncbi.nlm.nih.gov/23737049/
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.