Gasoline is primarily used as an engine fuel for vehicles, and as a product that is sold on the retail market, it must meet specific specifications in accordance with local or global legislation.
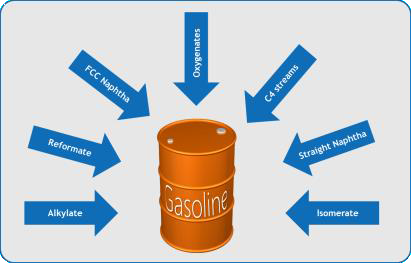
Image Credit: PAC L.P.
The product quality criteria, as detailed in the specifications, are typically comprised of boiling point, octane number, RVP, and sulfur concentration. These specifications relate to drivability and combustion characteristics as well as environmental factors.
At fuel stations, gasoline is generally dispensed across different grades, such as regular or premium. The grades are related to the octane rating, which indicates the antiknock properties of the gasoline.
Additionally, the majority of the gasoline sold today incorporates oxygenated components (“oxygenates”) such as ethanol, ETBE, or TAME. The oxygenates are introduced to match renewable fuel standards intended to limit greenhouse gas emissions as well as boost the octane rating.
Finished gasoline is a “blend product” comprised of straight, converted and other various “upgraded” refinery products, including naphtha, alkylate, isomerate, FCC naphtha, etc. and typically has a carbon number range from C4 to C11. Each hydrocarbon (group) has its own features in terms of octane number, vapor pressure, etc.
This makes the process of blending a challenge: the product should match the required specifications at the lowest possible cost.
Predicated on this carbon number range and the intermediate refinery products used, it is appropriate to conclude that gasoline could incorporate over 1000 components at concentrations ranging from trace level up to 10-15% per component.
Given the characteristics detailed in the product specification, in addition to the control of all process stages of the refining and blending processes, an accurate and reliable quantitative determination of the various hydrocarbon groups is vital.
This grouptype analysis can be challenging when taking into account the potential numbers of components and their diverse concentration range. The AC Reformulyzer® is the only single analysis method available that can separate the full gasoline in the different groups by carbon number.
This technique is the preferred method in contrast to single component analysis, where components may go undetected because of their low concentration or even be misidentified due to a lack of separation.
Analysis Techniques
Gasoline group type analysis is to be determined as the quantitative identification of saturates (divided by cyclic & straight/branched), olefins (divided by cyclic & straight/branched), aromatics, and oxygenates, all listed either by carbon number or as total.
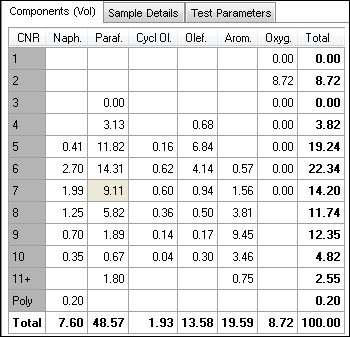
Figure 1. Typical gasoline analysis grouptype report. Image Credit: PAC L.P.
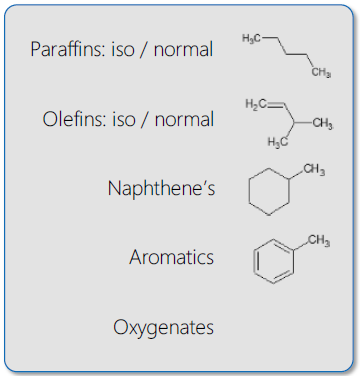
Image Credit: PAC L.P.
The intention of the analysis is not to determine individual hydrocarbon components (except benzene, toluene, and oxygenates). See figure 1 as an example. There are several chromatography methods that can be used to establish the group type composition of the gasoline:
- Single groups/components: These are methods that can analyze a single or distinct group of components and not the whole composition. For instance, frequently used methods include ASTM D3606, D4815 and D5580. As these methods do not report the full sample composition, a series of different analyses are required.
- Methods that (try to) analyze every individual component in a sample, such as DHA. Group type concentrations are quantified by adding each of the individual components identified. While a high-resolution column has a greater resolving power and a higher peak capacity, a central source of error in this method is the coelution of compounds of various classes.
- It is clear that there is ample overlap of peaks of varying classes, specifically if the sample is made up of a relatively high concentration of olefins.
- Single column analysis with the application of specialized detection techniques to circumvent separation and/or identification issues. These so-called hyphenated systems (single column separation with correlation-based detection) may enhance identification, but typically:
- Have issues with sensitivity and linearity. Therefore they cannot be relied on for quantifying components at low concentrations or run out of linearity for higher concentrations.
- Have response factors that deviate for the various hydrocarbon groups or even individual components, which affects quantification considerably in case of misidentification or unresolved coelutions.
- Only partially resolves coelutions.
- Rely on the completeness of the database used, thereby potentially missing components (such as certain oxygenates) in the overall quantification.
These shortfalls will result in an overall inaccurate grouptype analysis.
- “Real” group type analysis via the application of multi-dimensional gas chromatography, as utilized in the AC Reformulyzer. The system incorporates simple and robust separation principals, including:
- Aromatics - non-aromatic separation utilizing a polar column.
- Aromatics and alcohol separation predicated on boiling point (using a methyl-silicon column).
- Carbon number and cyclic/paraffinic separation with the application of a molecular sieve column.
These are simple separations with a limited risk of coelutions or misidentification. Figure 2 displays the typical separations that occur in systems.
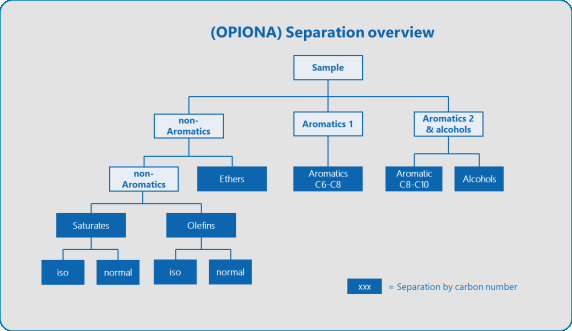
Figure 2. Typical gasoline analysis grouptype report. Image Credit: PAC L.P.
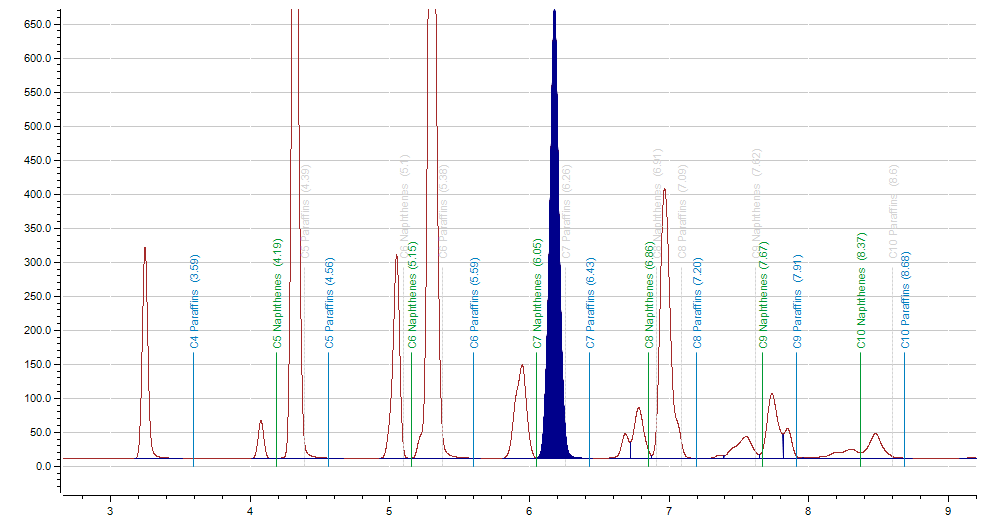
Figure 3. Example of separation for the saturated cyclic and paraffinic hydrocarbons by carbon number. Image Credit: PAC L.P.
Group Type Analysis Advantages
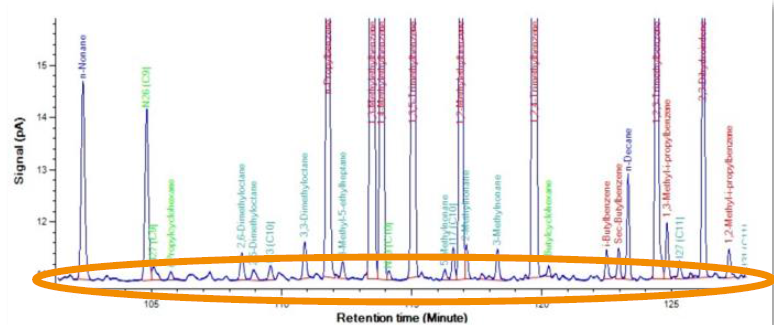
Figure 4. Typical ASTM D6730 DHA chromatogram, showing a huge number of components being neglected, and thus missing in the quantification. Image Credit: PAC L.P.
Utilizing a true group type analysis, such as the Reformulyzer, has a number of advantages over alternative techniques:
- As specified, gasoline can contain a significant number of components across a wide concentration range. There are advantages to be gained when separating the components by group and carbon number, such as:
- Components with low concentrations are not overlooked in the final results (see figure 4)
- It is not necessary to separate and identify each individual component, thus limiting the risk of coelution and/or related misidentification
- One analysis is able to cover the entire grouptype composition. There is no requirement for multiple analyses followed by a calculation of the results. The resulting accuracy for the full grouptype composition is predicated on a single analysis and not the sum total of errors of the various analysis required to generate just one result.
- The industry-standard FID detection offers sturdy, sensitive, and extremely linear detection of all eluted groups. The FID is not concentration sensitive, and the ions detected correspond to the concentration of the organic species. Quantification is predicated on theoretical response factors. Oxygenate FID factors are experimentally determined.
- The results of PTP programs demonstrate exceptional comparison with other direct measurement techniques, which is indicated by the various correlations as detailed in the ASTM D6839. The Reformulyzer typically demonstrates no or only an extremely limited bias with methods such as ASTM D5769, D5580, D3606 & D6550. Correlations can be accessed when needing to correct a small bias.
- The Reformulyzer falls into line with industry trends and has the complete capacity to analyze complex mixtures of oxygenates that could be present in high concentrations in today’s gasoline. This includes alcohols (such as methanol, ethanol & tertButylalcohol) as well as an extensive range of ethers (like MTBE, ETBE, TAME and TAEE) at concentrations up to ≈15% per component.
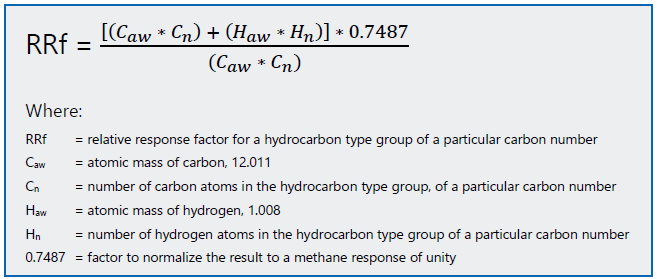
Figure 5. Relative Response Factor calculation based on chemical composition. Image Credit: PAC L.P.
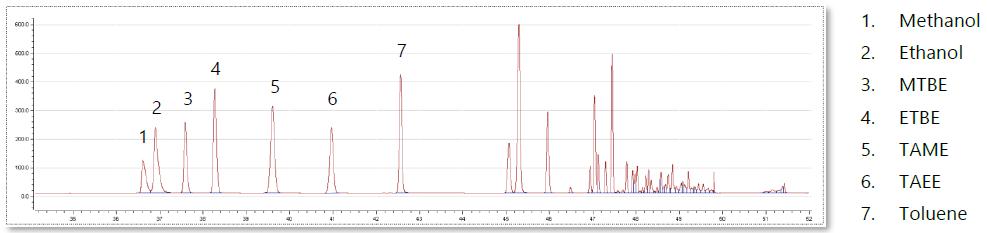
Figure 6. Oxygenates analysis on the Reformulyzer. Image Credit: PAC L.P.
Conclusion
The AC Reformulyzer M4® has established itself as the only true grouptype analyzer for complete gasoline composition analysis.
Through the application of straightforward and proven separation principles as well as a Flame Ionisation Detector, it is a reliable and direct detection method without sample or component-specific bias.
The AC Reformulyzer M4® is in operational use globally and is listed as the primary reference method for hydrocarbon type analysis in the European gasoline specification.
Since the first introduction of the Reformulyer in the mid-1990s, as a successor of the PIONA, numerous refineries and independent test laboratories still rely on the 4th generation of the Reformulyzer for true group analysis.
This is not just the analysis of only finished gasoline but also gasoline feedstocks. Subsequently, refineries are confident that they are reporting correct results using the only true grouptype analyzer on the market today.

This information has been sourced, reviewed and adapted from materials provided by PAC L.P.
For more information on this source, please visit PAC L.P.