The basic physical process of irradiating a material with photons of a known energy and measuring the ejected photoelectrons has remained unchanged over six decades since it was first commercialized.
X-ray photoelectron spectroscopy (XPS), also referred to as Electron Spectroscopy for Chemical Analysis (ESCA) is an invaluable tool for materials surface characterization. However, the capability and performance of a modern spectrometer have changed beyond recognition from those earliest instruments.
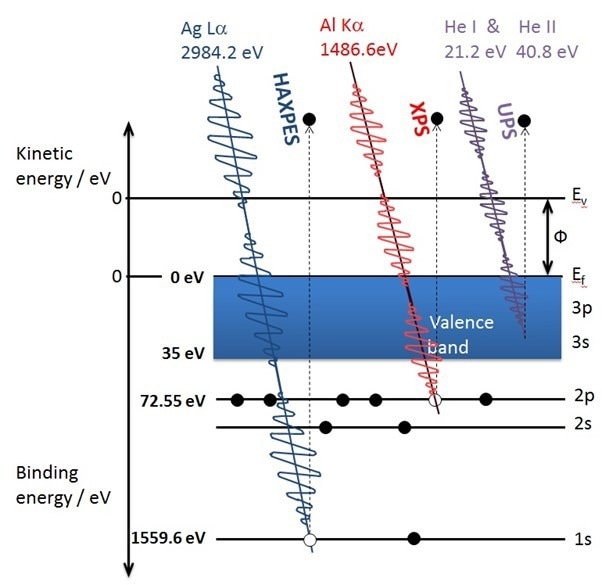
Figure1: Schematic diagram of commonly used photon sources interacting with the electronic ‘shells’ of aluminum to generate photoelectrons. Credit: Kratos Analytical
The latest generation of spectrometers is fully automated, allowing remote operation. Spectra can be acquired in just a few minutes. Chemical state images are acquired with micron spatial resolution over a few hundred-micron field of view. And recently developed cluster ion sources allow sputter depth profiling through microns of polymer material with retention of chemistry. And these are only some of the attributes that demonstrate the value of XPS for materials characterization.
XPS is a mature and widely used technique for probing the surface chemistry of a material. Instruments are used in both academic and industrial research labs. AXIS Supra user, Dr. Josh Lipton-Duffin from QUT, Brisbane explains ‘from a mechanistic view surfaces are where the action happens – the way in which materials interact is via their surfaces. All fields of chemistry, catalysis, epitaxy, and even biology to an extent are inherently surface-driven. It is important to have tools to study surfaces, because the physics is simply different on a surface than it is in the bulk of a material. Everyone understands surface-to-volume ratio goes up as dimensions shrink, and by extension, the trend towards miniaturization means that surfaces and interfaces are more important than ever before.
It is important to understand the origin of the surface sensitivity of XPS. The X-rays used are relatively ‘soft’ and can only excite photoelectrons whose binding energy within their electronic shells is less than the photon energy ca. 1500 eV. These low-energy photoelectrons cannot travel very far through a material before losing energy or being scattered and not detected. For a conventional lab. based XPS the information depth of the technique is widely accepted as 10 nm. As Dr. Andreas Holländer, Senior Scientist at The Fraunhofer Institute for Applied Polymer Research, states ‘Some important properties of materials such as the wetting behavior, the adhesion of glues, inks, paints, also of bacteria and viruses are determined by the outermost atomic layers of a surface’. This is expanded on by Martin Moos, Product Development Specialist at 3M, Germany who explains ‘The way in which materials interact with each other is through their surfaces and interfaces and hence this sample regime is crucial for their performance in any given environment. Adhesives are a practical example. They can be so strong that they can hold a structural panel for the life of a building or so gentle they can be applied to human skin to secure tubing. Applying an adhesive to an ill-prepared or contaminated surface will lead to compromised adhesion and ultimately bond failure. That is a common example where surface analysis comes into play.’
The ability to probe the lateral distribution of elemental and chemical states across a surface is provided by modern imaging XPS instruments. Determining the inhomogeneity of surface chemistry can be vital in understanding the functionality of a material or product. XPS imaging has been used to characterize corrosion pits, determining the chemical states present allowing a proposed reaction pathway. Dr. Mark Biesinger explains that Kratos XPS instruments at Surface Science Western, University of Western Ontario are ‘heavily involved in research on the copper-coated steel containers that will be used to store nuclear fuel waste in the deep geologic repository for the next million years or so. A lot of the work I’ve done on characterizing copper and other transition metal species by XPS has been driven by this research and other research in the nuclear energy field.’
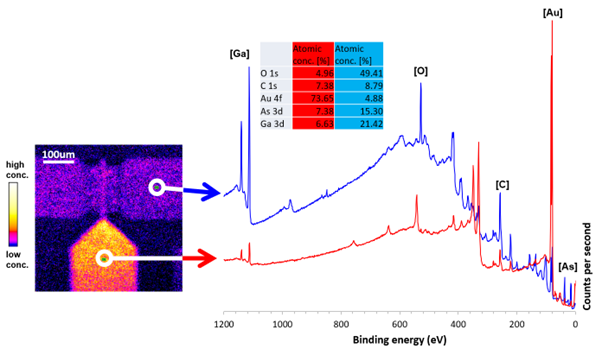
Image credit: Kratos Analytical
As highlighted by our Users, the value of XPS in providing quantitative, chemical information from the surface of a material is clear. Whether it’s increased publication output for academic Users or improvements in product quality for industrial Users, XPS instrumentation is an invaluable tool for a materials characterization lab.