In this Interview AZoM talks to Venkat Nandivada about adhesives evaluation for hydrochloric acid resistance.
What are some of the factors that should be considered when selecting an adhesive for chemical resistance?
Some of the factors to consider include the nature of the exposure to the chemical, duration of exposure, the temperature of the exposure, the specific chemical involved, the concentration of the chemical, and the frequency of exposure to the chemical.
The type of exposure, which can range from a splash to continuous immersion, will also play a role. Finally, chemical resistance can vary substantially under different mechanical and thermal loads.
What type of adhesive should be considered for applications where chemical resistance is required?
The first thing to understand about adhesives and chemicals is that there is no single adhesive that is the best choice for every chemical environment. It is true that some adhesive families are known for having a broad resistance to many types of chemicals.
Epoxies clearly lead the pack in this regard. Polyurethanes, silicones, UV curables, and polysulfides can all provide acceptable chemical resistance against a more limited range of chemicals. However, they cannot stand up to nearly as many chemical environments as epoxies.
It is better not to generalize, as adhesive chemistries can vary substantially, even within a family. Individual grades of adhesives can have different functional additives and curing agents that will affect their ability to withstand chemicals. Moreover, there are thousands of combinations of substrate materials, adhesives, and chemical agents to consider.
How does Master Bond test its adhesives for chemical resistance?
Many Master Bond epoxy systems are formulated for applications requiring superior chemical resistance. We continually test our materials by exposing them to specific chemicals over a long period of time. A common way of testing the chemical compatibility of an epoxy is by immersing a cured sample in a chemical and measuring its weight change over time.
A significant loss or gain in weight would indicate a decreased ability of a material to stand up to chemical exposure. These tests allow us to more accurately recommend the right product based on specific application requirements.
What was the rationale for testing resistance to Hydrochloric Acid?
Right from Oil and Gas to Medical to Aerospace industries, there is a need to resist hydrochloric acid in many applications. This acid is also one of the more aggressive chemicals for materials to handle, and there is often a need for protective coatings, sealants, and adhesives for such cases.
The compounds that Master Bond used for testing are a variety of two-component and one-component epoxies and a one-component UV curing system with good overall chemical resistance.
What concentration of HCl did you test?
The first round of testing involved exposure to 15% hydrochloric acid. We tested seven products; UV25, EP41S-5, EP62-1HT, EP21ARHT, EP17HT, EP125, and EP62-1BF. For the second round of testing, which involved exposure to 25% hydrochloric acid, the same products were tested.
For both tests, these products were compared against the resistance of a generic or standard non-chemically resistant epoxy. A few thin castings were made for each product and cured in accordance with their specifications.
Once the cured samples were created and the initial weight was recorded, the castings were immersed in either 15% or 25% hydrochloric acid. Then, we continued recording frequent weight measurements.
The charts below record the results of soaking for more than one year in each concentration. The cured samples were weighed periodically and the graphs demonstrate the percentage of weight change over time.
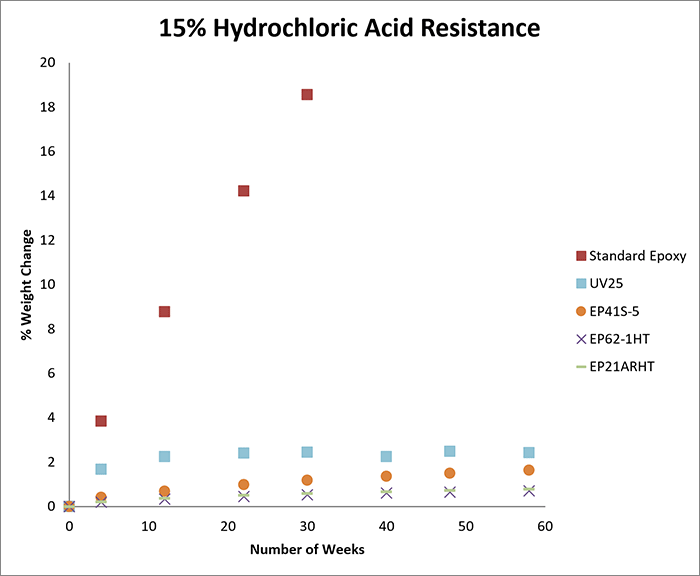
Image Credit: Master Bond Inc.
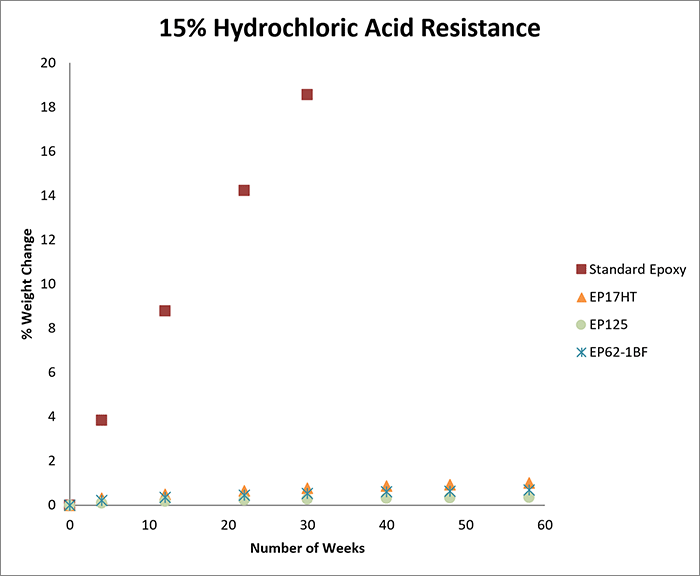
Image Credit: Master Bond Inc.
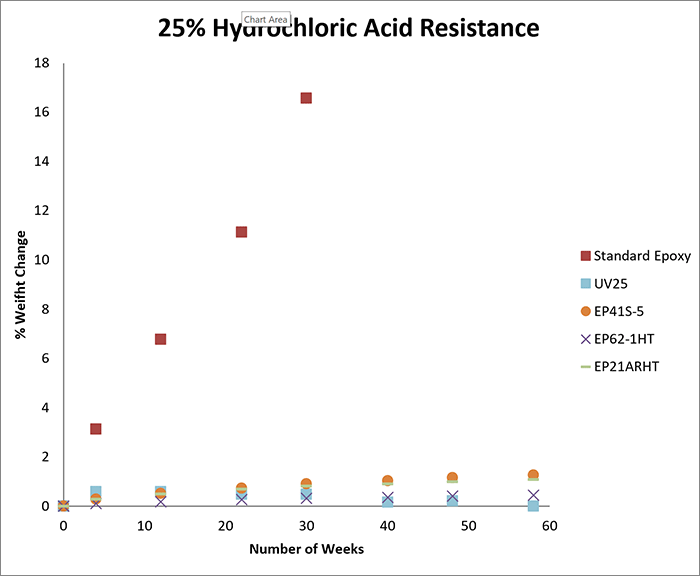
Image Credit: Master Bond Inc.
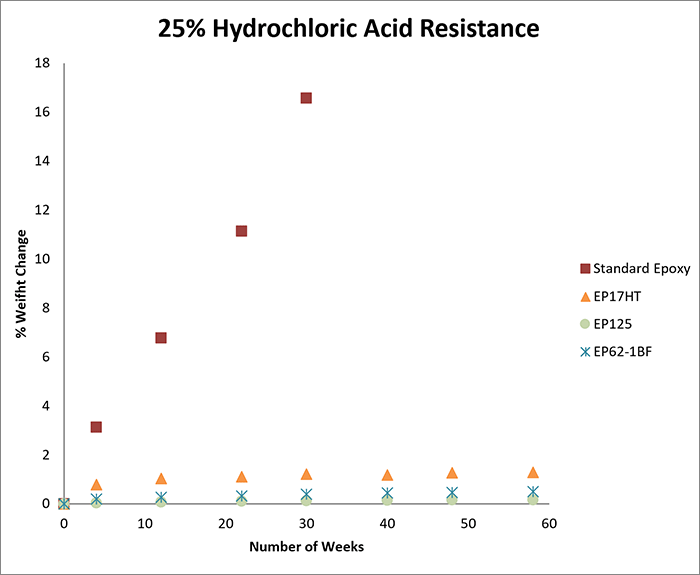
Image Credit: Master Bond Inc.
How should one interpret the results of the HCl chemical resistance tests?
As a benchmark for each concentration, we tested a casting of a standard epoxy under the same conditions as above. As shown in the graphs, the standard epoxy was markedly less resistant to hydrochloric acid than the other epoxies tested.
The standard epoxy demonstrated a significantly greater change in weight over time, and at both 15% and 25% concentration levels, the casting ultimately dissolved in hydrochloric acid after approximately seven months.
If an acid etches the surface of an epoxy, it results in a weight loss. If an acid causes the sample to swell, it results in a weight gain. In general, a weight change of less than 4-5% (gain or loss) can be considered excellent, especially since these tests may be more rigorous compared to actual service conditions.
It is also worth noting that in the context of a bonded joint or a potted assembly, the exposure to hydrochloric acid might not be as severe or direct as in the above test conditions.
How do you choose the right epoxy for an application where the resistance to hydrochloric acid is critical?
Many other factors must be considered in addition to the chemical resistance. Depending on the concentration level of hydrochloric acid, each of the epoxies in the charts above offers a distinct set of performance properties.
For example, at both 15% and 25% concentrations, if electrical insulation and low outgassing are needed, EP41S-5 may be a good option. If a moderate viscosity and long working life are needed, EP62-1HT can be considered. If a one-part system is needed at either concentration, EP17HT is an excellent candidate.
These products all need to be processed or cured differently, but the key in all cases is the addition of heat for enhancing/optimizing chemical resistance, especially against hydrochloric acid.
About Venkat Nandivada
Venkat Nandivada is a Manager of Technical Support at Master Bond. He has a Masters in Chemical Engineering from Carnegie Mellon University. He analyzes application oriented issues and provides product solutions for companies in the aerospace, electronics, medical, optical, OEM and oil/chemical industries.

This information has been sourced, reviewed and adapted from materials provided by Master Bond Inc..
For more information on this source, please visit Master Bond Inc..
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.