Isotopic analysis primarily involves the separation of isotopes based on the significant differences in mass. The method is effective for identifying the isotopic signature within the inorganic and organic compounds.

Image Credit: remotevfx.com/Shutterstock.com
Isotopes of an element can be differentiated by the variations in the number of neutrons. In the periodic table, only 21 elements possess a single naturally occurring isotope form/are monoisotopic. In an element, the stable isotope forms often include one or more rare isotopes and one common isotope. For instance, 98.89% of the element carbon (C) is the stable isotope 12C while 1.11% is the stable isotope 13C.
The superscript represents the sum of neutrons and protons. For instance, 12C has six neutrons and six protons, while 13C possesses an extra neutron and is slightly heavier than the 12C isotope. Stable isotopes do not decay over time as radioactive isotopes. In non-metal elements such as sulfur, oxygen, C, and nitrogen (N), the isotopic compositions are typically expressed as rare to common stable isotope ratio (R), such as 13C/12C, 15N/14N.
Additionally, the delta (δ) notation is used to express the deviation of the sample R from a reference standard in parts per thousand (‰) as R is small for most stable isotope ions and the differences measured in isotope ratios at natural abundance are also small. For instance, the isotopic composition of 2H/1H in a sample is expressed as the δ2H value, while the isotopic composition of 18O/16O in a sample is expressed as the δ18O value.
Reference standards for the non-metal elements include Vienna-Standard Mean Ocean Water (VSMOW) for hydrogen and oxygen, Vienna-Pee Dee Belemnite (VPDB) for C and oxygen, atmospheric nitrogen (AIR) for N, and Vienna-Canyon Diablo Troilite (VCDT) for sulfur.
Isotopic analysis is performed using a mass spectrometer or an emission spectrometer. However, the sample must be converted to a gas before performing isotopic analysis using these instruments. Isotopic analysis is used in different research fields to obtain important information concerning biological and geological activities, ancient climate, origin, and age.
Methods Used for Isotopic Analysis
Isotope ratio mass spectrometry is a specialized method used to obtain information about the biological, chemical, and geographic origins of substances. The isotope ratios of elements such as N, C, oxygen, hydrogen, and sulfur can become locally depleted or enriched due to different thermodynamic and kinetic factors.
Thus, the isotope ratio measurement can be utilized to differentiate samples that share similar chemical compositions. For instance, isotope mass spectrometers can separate and quantify different N2 gas molecules depending on their behavior when these molecules are accelerated through a magnetic field.
Emission spectrometers are also suitable for highly accurate isotopic analysis. These spectrometers are portable, cheaper to maintain and purchase, and require smaller samples for analysis. However, emission spectrometers have been gradually replaced by better and bigger instruments.
Applications of Isotopic Analysis
Isotopic analysis is used in chemistry, archaeology, forensic science, oceanography, and physical anthropology. Conventionally, isotopic analysis is used in the study of hydrological, geological, and ecological systems.
In archaeology, C isotopes are analyzed to determine the C source at the base of the food chain. Archaeologists use stable isotope analysis to obtain information from the bones of an animal to identify the photosynthesis process of the plants consumed by the animal during its lifetime.
In forensic sciences, isotopic analysis is increasingly becoming an important tool for several applications, such as for the analysis of different forms of trace evidence such as soils and paints and explosives, authentication and sourcing of food and beverages, and provenancing unidentified human remains and poached wildlife.
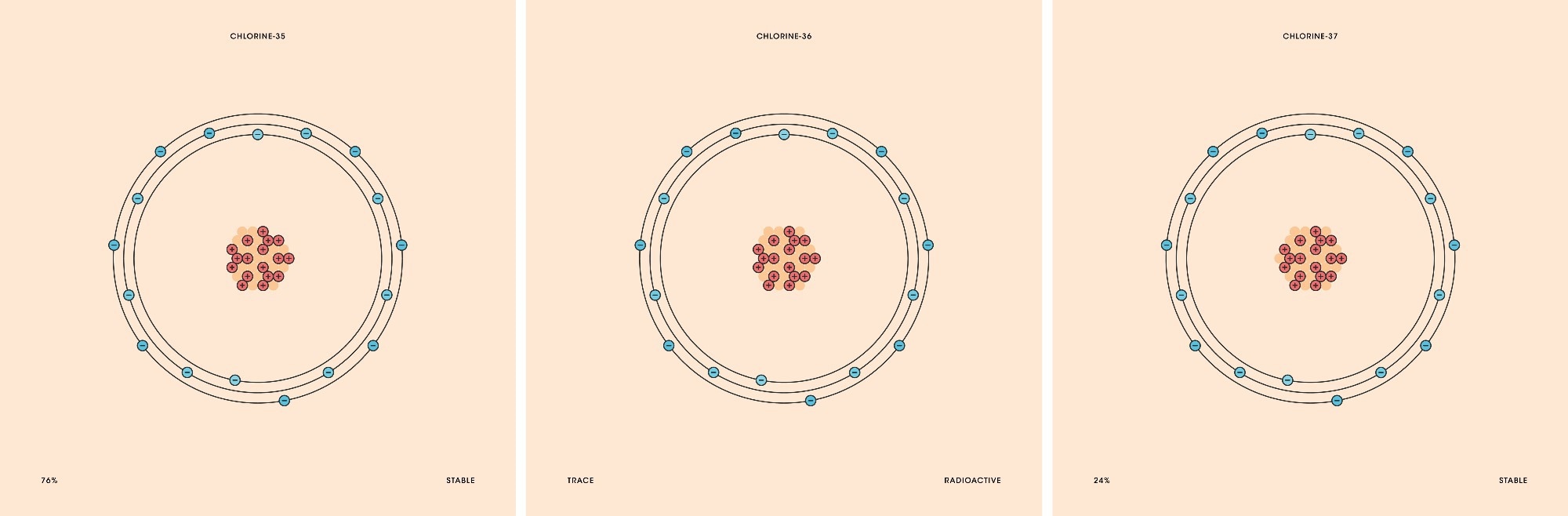
Chlorine and its isotopes. Image Credit: Patricia F. Carvalho/Shutterstock.com
Modern isotopic analyses of human remains have integrated the use of multi-isotope profiles and isotopic landscapes from several body tissues such as teeth, hair, nails, and bone to identify the region of origin of unidentified human remains.
Limitations of Isotopic Analysis
The use of mass spectrometry often leads to sample destruction, which necessitates the identification of a non-destructive method to determine the isotopic ratio, specifically for the analysis of precious samples. Moreover, isotopic ratio mass spectrometry only gives the global δ13C and requires a high purity compound while determining the δ13C values.
New Studies Using Isotopic Analysis
In a study recently published in the journal Chemosphere, researchers used compound-specific isotope analysis to identify the degradation mechanism during the photodegradation of diethyl phthalate using nano titanium dioxide as a catalyst.
Titanium dioxide has gained considerable attention for application as a photosensitizer in advanced oxidation processes and wastewater treatment. The diethyl phthalate degradation process was described with first-order kinetics in the applied concentration ranges.
In the ultraviolet/nano-titanium dioxide system, the diethyl phthalate degradation rate was fastest under neutral conditions and slowest under alkaline conditions. The 2H and 13C isotope fractionation triggered by the photodegradation of diethyl phthalate at pH 11, 3, and 7 yielded normal isotope effects.
Hydroxyl radicals were the dominant free radicals in both titanium dioxide/hydrogen peroxide/ultraviolet/diethyl phthalate and titanium dioxide/ultraviolet/diethyl phthalate systems and all pH conditions. Moreover, the 13C and 2H fractionation indicated that the hydroxyl ion addition on the diethyl phthalate benzene ring was the main conversion pathway.
Thus, the findings of this study demonstrated that compound-specific isotopic analysis can be a feasible method to identify the diethyl phthalate reaction pathways for applications such as wastewater treatment.
To summarize, isotopic analysis will remain a crucial method for different existing applications. Specifically, non-destructive isotopic analysis methods, such as analysis using muonic X-ray measurement, will gain more prominence in the future as they can be applied to any element without damaging the sample. However, more research is required to improve the accuracy of these non-destructive methods.
More from AZoM: A Closer Look at Semiconductor Test Equipment
References and Further Reading
Richnow, H.H., Cao, Y., Zhu, J., Pang, W., Li, H., Made, M., Yao, J., Min, M. (2022). Compound-specific isotopic analysis to characterize the photocatalytic reaction of TiO2 nanoparticles with diethyl phthalate. Chemosphere, 307, 4. ISSN 0045-6535. https://doi.org/10.1016/j.chemosphere.2022.135892
Gilbert, A., Silvestre, V., Robins, R.J., Remaud, G. S. (2012). Biochemical and physiological determinants of intramolecular isotope patterns in sucrose from C3, C4 and CAM plants accessed by isotopic 13C NMR spectrometry: A viewpoint. Natural Product Reports, 29(4), 476-86. http://dx.doi.org/10.1039/c2np00089j
Kubo, K. M., Shinohara, A., Miyake, Y., Tampo, M., Kawai, Y., Terada, K., Strasser, P., Kudo, T., Ninomiya, K. (2019). Development of non-destructive isotopic analysis methods using muon beams and their application to the analysis of lead. Journal of Radioanalytical and Nuclear Chemistry, 320, 801–805. https://doi.org/10.1007/s10967-019-06506-9
Muccio, Z., Jackson, G.P. (2009). Isotope Ratio Mass Spectrometry. Analyst, 134(2), 213-22. https://doi.org/10.1039/b808232d
Bartelink, E.J., Chesson, L.A. (2019). Recent applications of isotope analysis to forensic anthropology. Forensic Sciences Research, 4(1):29-44. https://doi.org/10.1080%2F20961790.2018.1549527
Fernandes, R., Jaouen, K. (2017). Isotopes in archaeology. Archaeological and Anthropological Sciences, 9, 1305–1306. https://doi.org/10.1007/s12520-017-0507-4
McCarthy, M. D., Bronk, D. A. (2008). Analytical Methods for the Study of Nitrogen. Nitrogen in the Marine Environment, 2nd Edition, 1219-1275. http://dx.doi.org/10.1016/B978-0-12-372522-6.00028-1
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.