The lowest levels of critical purity are offered by Air Products BIP® gas cylinders, making them the preferred choice for the most demanding research and industrial applications. This article explores a range of functions for which these BIP® gases can be used and examines what sets them apart from other ultra-high-purity gases.
How Important is Purity?
A time-old question within the industry is: ‘How pure is “pure”?’. The application defines the required purity. For instance, pure oxygen (99.5%) is commonly mixed with fuels such as propane in cutting and welding.
To improve their shelf-life, pharmaceutical products are often packaged in an atmosphere of pure nitrogen. Pure carbon dioxide is widely used as an additive to carbonate drinks in the beverages industry. EU regulations enable up to 1% total impurities in gases such as CO2 for food and beverage applications.1
By contrast, manufacturers in the chemical or pharmaceutical industries may have much tighter purity requirements for processes where impurities could risk causing serious negative effects.
However, some of the applications that have the highest requirements for purity can be found in semiconducor manufacturing and analytical chemistry, with techniques like gas chromatography. Even very small levels of impurities, within these applications (especially the likes of oxygen, water vapor or hydrocarbons) can damage the quality of products or result in incorrect analyses.
Air Products offers a unique solution for these applications: BIP® gases.
The BIP® range has been specially formulated to offer the lowest levels of absolute purity (minimum 99.999%) and specifically reduce critical impurities such as O2, H2O, and hydrocarbons.
Applications of Ultra-High-Purity Gases
Semiconductor Fabrication
As microelectronics fabrication can be sensitive to contaminants, the semiconductor industry is a major consumer of ultra-high-purity gases for almost every step of the manufacturing process.2
Nitrogen, oxygen, helium, and hydrogen are used for cleaning procedures, and some chemically reactive specialty gases are used for processes such as etching.2
Over the last 50 years, improvements in semiconductor performance can mainly be attributed to a significant increase in the number of transistors squeezed into a given volume. However, semiconductor fragility and sensitivity to contamination have increased as their devices' critical dimensions have decreased. The purity of any gases used in semiconductor manufacturing is critical.
Image Credit: aslysun/Shutterstock.com
Analytical Chemistry
Analytical chemistry is primarily concerned with the molecular study of what substances and samples are composed of. For instance, the identification and quantification of impurities in gas mixtures are enabled by analytical chemistry techniques. By design, analytical chemistry techniques are acutely sensitive to impurities of any kind. However, for techniques that depend on gases, this can introduce problems for techniques, either as plasma gas (such as in inductively coupled plasma spectroscopy)3 or as carrier gases (such as in gas chromatography). The presence of unwanted impurities in carrier/plasma gases introduces noise, making it difficult to discern what comes from the sample and the gas. A reduction in baseline noise, an improvement in peak separation, and an extension of equipment lifespan can all occur after using ultra-high purity gases in analytical applications.
Beyond Purity: BIP® Gases Provide Ultra-low Critical Impurities for the Most Demanding Applications
While purity is vital in assessing gas quality, it is not the only important factor. For instance, gas with a purity grade of 6.0 offers a purity of 99.9999%. The composition of the remaining 0.0001% can make the difference between success and failure in demanding research or semiconductor applications.
Air Products can boast ultra-high levels of purity with its BIP® range. They can also guarantee the lowest levels of critical impurities available anywhere on the market: oxygen (≤10 ppb), moisture (≤ 20 ppb) and total hydrocarbons (< 100 ppb).
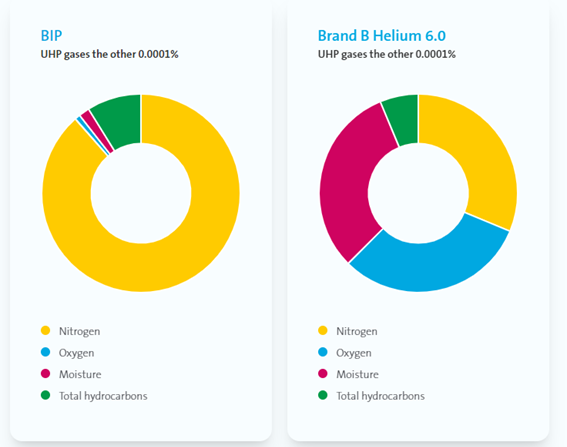
Image Credit: Air Products PLC
Using unique integrated filter technology, low levels of critical impurities are achieved, cutting down on residual oxygen, moisture, halocarbons, hydrocarbons, carbon monoxide, and carbon dioxide at the point of use. BIP® cylinders guarantee the highest possible purity level and eliminate the need for external purification equipment, enabling many of Air Products’ customers to make significant cost savings.
Customers can get in touch with a member of the Air Products team to learn more about its BIP® range of gases.
References and Further Reading
- Commission Regulation (EU) No 231/2012. http://data.europa.eu/eli/reg/2012/231/oj/eng (2012).
- Funke, H. H., Grissom, B. L., McGrew, C. E., & Raynor, M. W. (2003). Techniques for the measurement of trace moisture in high-purity electronic specialty gases. Review of Scientific Instruments, 74(9), 3909–3933. https://doi.org/10.1063/1.1597939
- Patel, K., Patel, J., Patel, M., Rajput, G. & Patel, H. Introduction to hyphenated techniques and their applications in pharmacy. Pharm Methods 1, 2 (2010).

This information has been sourced, reviewed and adapted from materials provided by Air Products PLC.
For more information on this source, please visit Air Products PLC.