Sponsored by CSEMReviewed by Maria OsipovaMar 27 2023
The primary aim of the ELAINE project is to assess the effectiveness of using active dry electrodes for fetal ECG (fECG) monitoring. The project intends to replace the traditional method of monitoring the health status of an unborn through heartrate-based cardiotocography (CTG) with the measurement of an abdominal ECG.
It is anticipated that this new technology will offer greater reliability in monitoring fetal health status.
The system is founded on a technology that aims to decrease cabling complexity, consequently enhancing the comfort level of expecting mothers. This technology was originally developed as part of a contract with the European Space Agency (ESA) for the creation of a multi-signal wearable monitoring system.
The developed system is currently undergoing testing at the Maternity Ward of the University Hospital of Bern (Inselspital), located in Switzerland.
Implementing an unobtrusive electronic fetal monitoring (EFM) system utilizing wearable sensors can benefit from two main market pushes.
Firstly, there is an increasing demand among obstetricians to replace traditional cardiotocography (CTG) systems with an alternative that provides greater comfort to expectant mothers.
Secondly, the consumer market, particularly the booming industry of FemTech (i.e., female technology), presents a significant opportunity for the wearable EFM system. This sector comprises various areas, such as fertility solutions, period-tracking apps, pregnancy and nursing care, as well as reproductive system healthcare.
CTG is the most commonly used procedure to evaluate the fetal heart rate before and during labor. Despite its introduction in the late 1960s, this method's usefulness has been controversial.
In addition to the technical difficulties inherent to the method, including data analysis in adipose patients, active fetuses, or multiple gestations, confusion between fetal and maternal pulse, and assessment during water delivery, there is also a concern regarding the significant inter- and intra-observer variability resulting from the visual interpretation of CTG traces.
Furthermore, strong contractions or changing labor positions of the parturient can also pose a challenge.
Unreliable CTG interpretation can potentially result in unwarranted medical interventions, such as cesarean section and vaginal-operative deliveries.1
Over the last 15 years, CSEM has been dedicated to developing technologies for measuring multimodal physiological parameters. This has led to a new architecture for "cooperative sensors" based on active electrodes connected via a bus comprising only two unshielded wires.
As a result, this technology significantly simplifies the cabling complexity of the sensor system.2 The two unshielded wires serve a dual purpose, providing a common reference potential required for biopotential measurements and also enabling synchronization, data transfer, and powering of the sensors.
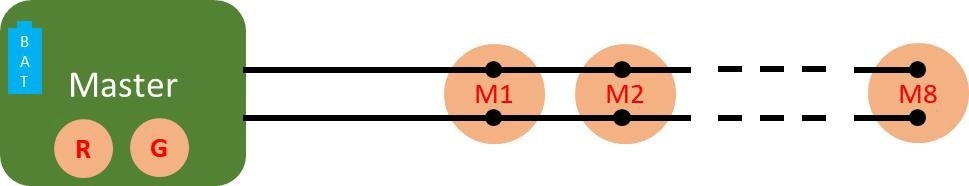
Figure 1. Connection diagram of the ELAINE system comprising a master unit, 8 cooperative sensors (M1 to M8), and a bus made of 2 unshielded wires connecting the master to the cooperative sensors. Image Credit: CSEM
comprises one master sensor and eight cooperative sensors. The master sensor features a refined architecture that creates a low-impedance path for current induced by electromagnetic disturbances. This eliminates the need for wire shielding to achieve high-quality ECG measurements.3
The low-impedance path is established through the utilization of two skin contacts, known as the guard and reference electrodes. The system is powered by a single battery integrated into the master sensor. The power is transmitted to the measuring sensors via the same two wires.
As demonstrated in Figure 2, the cooperative sensors are connected to conventional gel electrodes using a snap button to measure the biopotential during a trial. The signal is then amplified, filtered, and digitized directly within each cooperative sensor.
Subsequently, the digital data is transmitted to the master sensor via the bus and then wirelessly sent to an external unit. The measurements are ultimately processed to segregate the signal into two components: the maternal ECG (mECG) and fetal ECG (fECG).
Placing the analog front end and digitalization directly onto the electrode location increases the input impedance at the electrode and minimizes susceptibility to electromagnetic disturbances. Both of these features are crucial to improve the quality of the fECG signal.
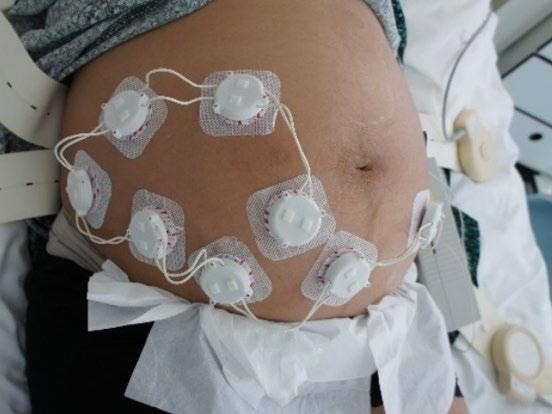
Figure 2. The ELAINE system measuring 8 fetal ECG leads on a pregnant patient at the Maternity Ward of the University Hospital of Bern (Bern Ethical Committee, Project ID: 2019-01899). Image Credit: CSEM
In conclusion, the proposed system enables the measurement of an 8-lead fECG on pregnant women with flexible positioning of the cooperative sensors and significantly reduces cabling complexity.
By measuring fECG instead of relying solely on heartrate-based cardiotocography (CTG), a greater level of reliability in monitoring the health status of the unborn fetus is anticipated.
ESA Technology Transfer Success Story
Dry Electrodes to Monitor Vital Signs: From Astronauts in Space to Fetuses in Utero
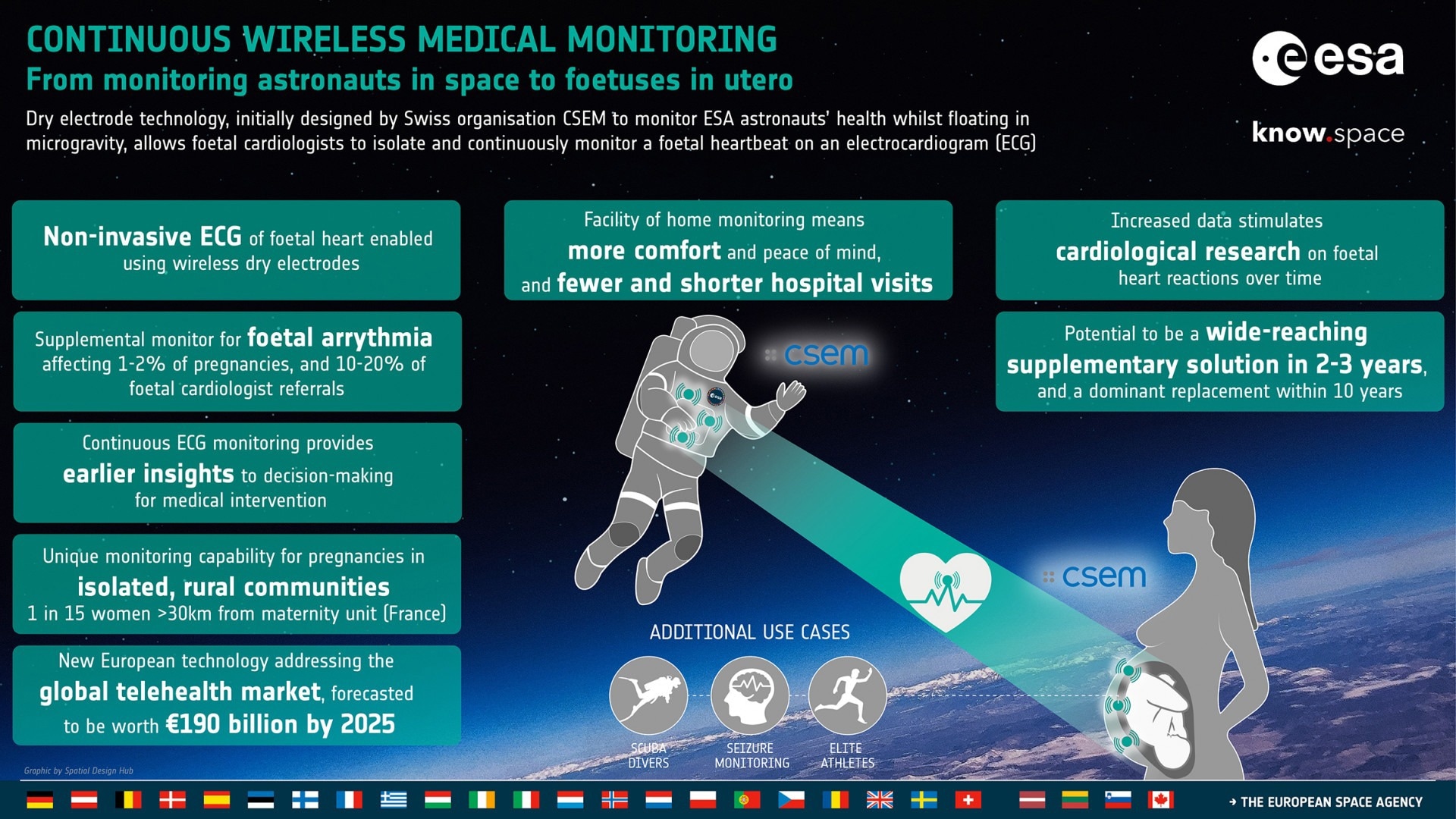
Image Credit: The European Space Agency
Initially designed by the Swiss organization CSEM to monitor ESA astronauts’ health whilst floating in microgravity (LTMS system), dry electrode technology enables fetal cardiologists to isolate and continuously monitor a fetal heartbeat on an electrocardiogram (ECG).
CSEM saw this as an opportunity to develop a technology (Electronic Foetal Monitoring System - ELAINE) using the active dry electrodes to provide a more comfortable wireless solution, using ECG technology rather than CTG’s ultrasound solution.
Under the ESA contract, CSEM came up with the LTMS system, which was initially intended to monitor the vital signs of astronauts using an easy-to-wear vest equipped with three dry electrodes.
This same technology is now being applied to fetal monitoring. Before labor, monitoring a fetus’ heartbeat is sometimes crucial to identify any abnormalities. However, during labor, regular monitoring of fetal cardiac activity is recommended.
ELAINE holds great promise in this regard, supplementing or even replacing traditional CTG machines in hospital environments. Furthermore, this technology could also pave the way for high-accuracy, at-home fetal monitoring.
In collaboration with the Maternity Ward of the University Hospital of Bern, CSEM is currently working on the development of a portable device4,5 that can be used at home by women to monitor babies. This device will also allow doctors and medical professionals to remotely monitor a fetus and call mothers into the hospital when abnormalities are detected.
There are about 140 million newborns per year worldwide. This is a considerable market, even if ELAINE addresses only a small portion of it. A commercialization effort through CSEM's Innovation Booster Grant is underway. The grant, awarded in 2022, covers initial marketing activities toward creating a start-up company.
Recent advancements in home monitoring mean a decreased risk of exposure to diseases such as COVID-19 for patients, fewer hospital visits, and shorter stays at the hospital.
A reliable home monitoring solution allowing a mother to check the fetal heartbeat to alleviate some of their concerns could decrease the number of times she would have to go to the hospital in person. It can also help decrease the length of time a woman must remain in the hospital for monitoring purposes.
Using active dry electrodes for ECG in fetal heart monitoring will become an impactful supplementary solution in two to three years and a potential replacement method within just ten years.
ELAINE can provide additional supplementary monitoring solutions for fetal arrhythmia, accounting for 1-2% of all pregnancies and 10-20% of all referrals to fetal cardiologists.
References
- Z. Zhao, et al., “A Comprehensive Feature Analysis of the Fetal Heart Rate Signal for the Intelligent Assessment of Fetal State,” J. Clin. Med. 2018 Aug 20; 7(8). pii: E223.
- M. Rapin, et al., “Wearable Sensors for Frequency-Multiplexed EIT and Multilead ECG Data Acquisition,” IEEE Trans. Biomed. Eng., vol. 66, no. 3, pp. 810–820, Mar. 2019.
- M. Rapin, et al., “Two-wire bus combining full duplex body-sensor network and multilead biopotential measurements,” IEEE Trans. Biomed. Eng., vol. 65, no. 1, pp. 113–122, Jan. 2018.
- F. Braun, et al., “Evaluation of a Wearable System for Fetal ECG Monitoring Using Cooperative Sensors,” presented at the EMBC 2022, Glasgow, Scotland, UK, Jul. 2022.
- A.-P. Radan et al., “Electronic fetal monitoring system (ELAINE): Novel technique for non-invasive assessment of the fetal electrocardiogram,” presented at the SGGG 2021, St. Gallen, Switzerland, Jun. 23, 2021.

This information has been sourced, reviewed and adapted from materials provided by CSEM.
For more information on this source, please visit CSEM.