Enhancing the performance of energy storage devices while simultaneously ensuring sustainability has been widely acknowledged as a major challenge for both the scientific community and industrial manufacturers.
Image Credit: Oxford Instruments Magnetic Resonance
The market for Lithium-ion batteries, which presently is the preferred battery technology for many different applications, is expected to reach $116 billion by 2030. For the upcoming generation of batteries to succeed, it is crucial to enhance energy and power densities, along with charge and discharge times, cost-effectiveness, longevity, and safety.
A schematic of the constituent parts of a conventional Lithium-ion battery is presented in Figure 1. The electrolyte, which carries the current, plays a crucial role in determining the performance, safety, and cost of commercial battery systems. However, it is often overlooked in favour of other components such as cathodes.
The mixture of different solvents, additives, and lithium salts contained within complex electrolyte systems must be finely tuned to ensure optimal performance and sustainability.
Therefore, it is imperative to employ accurate, quantitative, and dependable methods for characterizing and analyzing electrolytes throughout the entire process, from inspecting incoming raw materials to developing new formulations and ensuring quality control during high-volume production.
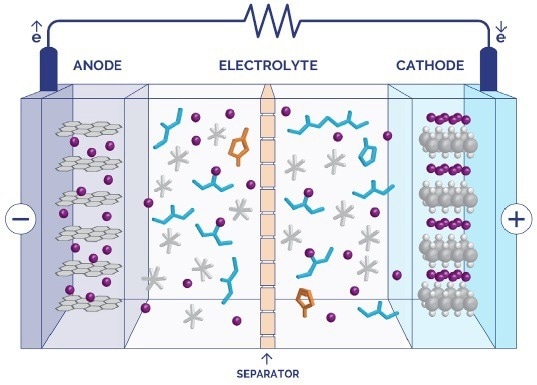
Figure 1. Schematic of a lithium-ion battery. Image Credit: Oxford Instruments Magnetic Resonance
Benchtop NMR Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy involves the use of a radio frequency pulse to investigate specific isotopes of elements present in the liquid sample while they are subjected to an external magnetic field.
This technique provides insights into the chemical structure, concentration, and local chemical environment of the probed nuclei in the sample, including electrolytes used in batteries. A comprehensive understanding of the analyte's structure can be obtained by probing a wide range of nuclei across various frequencies.
Broadband benchtop NMR spectroscopy is a unique tool that facilitates the qualitative and quantitative analysis of all components present in a complex electrolyte mixture. In the past, instruments were limited to only a few nuclei and had limitations in detecting numerous compounds.
Portable benchtop NMR instruments, operating at room temperature with permanent magnets, now remove accessibility barriers of large cryogenic NMR systems such as cost, maintenance, space, and skilled operators.
The ease of use makes portable benchtop NMR instruments ideal for tasks such as rapid development, reaction monitoring, and quality control, where obtaining quick and precise results is crucial for instant feedback and optimization purposes.
The Oxford Instruments X-Pulse benchtop NMR is the only genuine broadband spectrometer that allows researchers to investigate any electrolyte design and composition of their choice.
The X-Pulse benchtop NMR spectrometer can collect spectra from a wide range of nuclei including 1H and 19F nuclei on the high frequency channel. While, the broadband channel can excite and detect a wide range of nuclei, including 13C, 7Li, 31P, 11B, 29Si, 27Al, and 23Na, with a Larmor frequency between 25 and 11 MHz in a 1.4 Tesla magnetic field.
The X-Pulse benchtop NMR's capability to perform two-dimensional spectral methods with pulsed field gradients and shaped pulses enables researchers to conduct a range of advanced experiments to quantify specific chemical and physical properties that define electrolyte performance.
Battery Performance, Where Does NMR Fit?
Battery technology is often considered a balancing act between five key parameters.
Charge density refers to the amount of energy stored in a cell, while power density indicates the rate at which this energy can be extracted. Lifetime, or charge cycle lifetime, is a measure of how quickly the charge density reduces.
Safety is a critical consideration for battery performance throughout its life cycle, as is the cost concerning the amount of energy provided per unit of currency.
Today's materials research aims to maximize all of these parameters for single-battery chemistry. Figure 2 illustrates how some typical battery chemistries, defined by the cathode material, balance these parameters.
This article discusses how benchtop NMR can aid in the development of new electrolyte chemistries by providing quick insight into the physical and chemical properties that underlie critical performance parameters. It will also provide a detailed discussion of these parameters.
NMR is inherently quantitative and can measure ion concentration (such as Li+, Na+, etc.) which is crucial to energy density. It can also determine the solvent composition or the degree of degradation, which ensures safety. These measurements can be performed in seconds, which minimizes cost.
Using a quick set of measurements, the diffusion properties of electrolyte components, and therefore the ionic conductivity, can be calculated, allowing an understanding of potential power density as the chemistry is being developed.
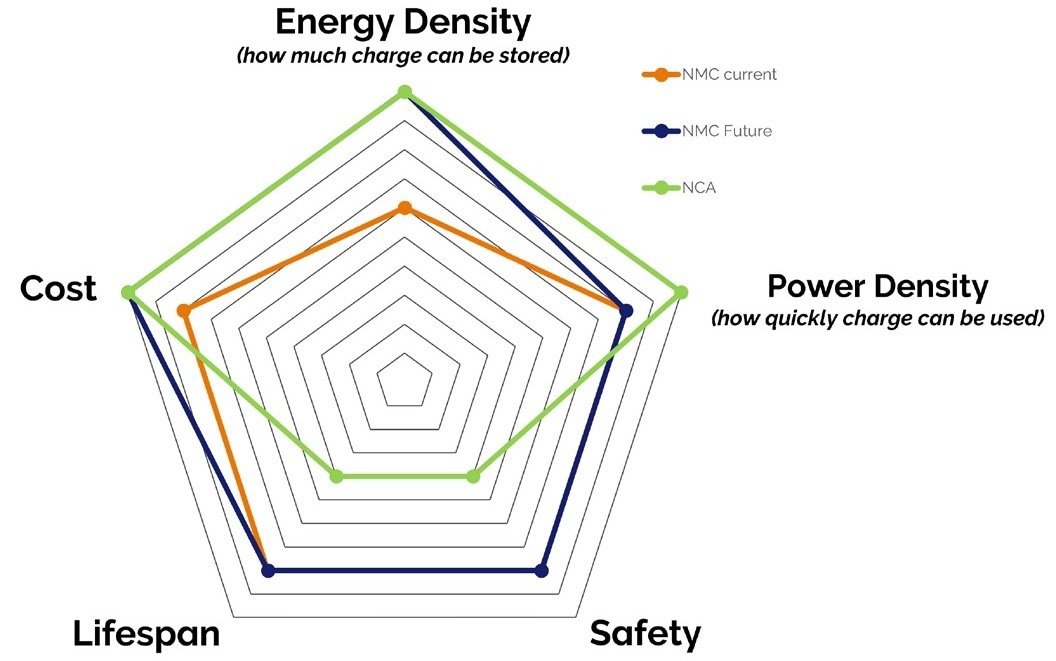
Figure 2. Chart showing the balance of critical parameters for 3 common battery chemistries. Image Credit: Oxford Instruments Magnetic Resonance
Rapid Assessment of Electrolyte Composition
For a non-aqueous electrolyte consisting of an organic solvent or a mixture of solvents, the solvents will be visible in a simple 1H NMR spectrum. Different chemical environments of 1H nuclei within the molecules produce distinct signals with characteristic chemical shifts.
Some signals may be split into doublets, triplets, or other multiplets, providing valuable information about the nature of other NMR active nuclei in the vicinity, such as the isotope (element), distance, and quantity.
A straightforward example of a spectrum similar to that can be found in Figure 3, where a 1H spectrum was taken of a mixture of common solvents present in Lithium-ion batteries, namely, ethylene carbonate (EC), dimethyl carbonate (DMC), and diethyl carbonate (DEC).
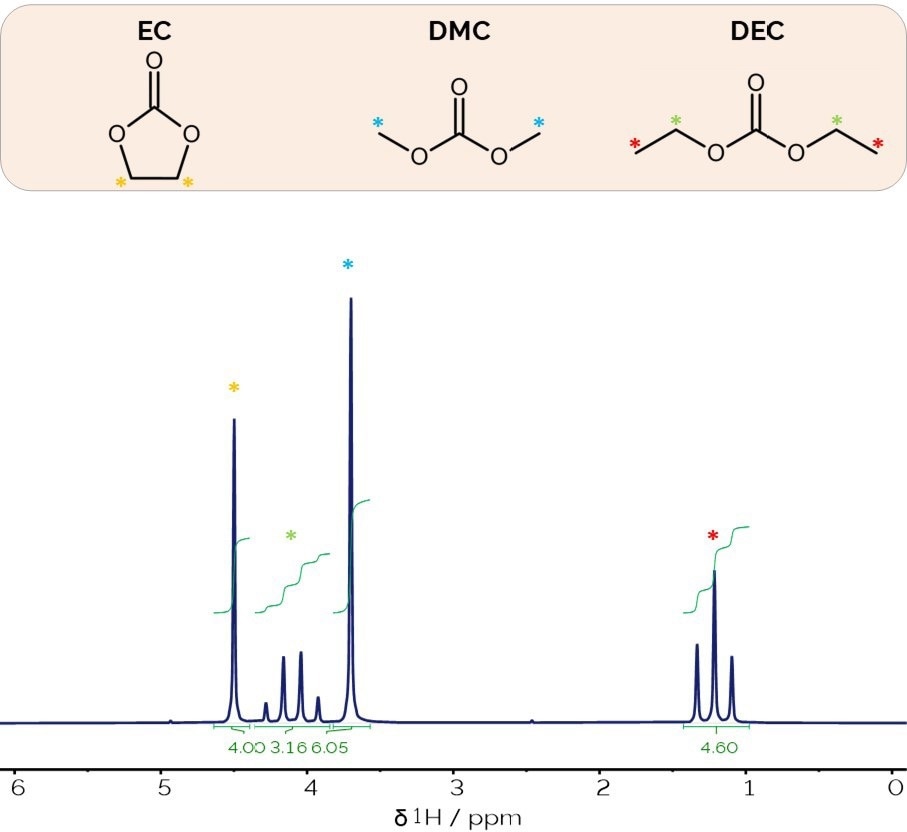
Figure 3. 1H spectrum of a simple solvent mixture typical for electrolytes. Image Credit: Oxford Instruments Magnetic Resonance
Distinct peaks were observed in the 1H spectrum of a mixture of solvents commonly found in lithium-ion batteries. EC and DMC exhibited one singlet each, while DEC showed two signals, with the CH3 group signal appearing as a triplet and the CH2 signal appearing as a quadruplet due to their respective splitting patterns.
This can be explained using the n+1 rule, which applies to spin 1H and other similar nuclei. According to this rule, a peak splits into n+1 signals if n equivalent NMR active nuclei are situated close enough to cause coupling.
In this case, the three protons in CH3 cause the splitting of the CH2 signal into n+1 = 4, while the CH2 group causes the CH3 position to be split into three. This type of splitting provides valuable information about the structure of unknown compounds.
The area under the peaks is proportional to the number of nuclei present, making it possible to quantify the components against each other or a standard. By integrating the peaks, a ratio of 1:1:1 by mass could be determined.
The same principles can also be applied to more complex mixtures and nuclei beyond 1H. Figure 4 displays multinuclear NMR spectra obtained from a commercial battery electrolyte.
All spectra exhibit well-defined and distinct signals for the species containing nuclei such as 13C, 19F, 31P, 11B, and 7Li. The n+1 rule remains applicable for 19F and 31P, even in the presence of coupling between different nuclei.
The 31P signal splitting into seven suggests the presence of six coupling nuclei, while the 19F signal at −69 ppm is split into two, indicating the presence of one coupling species. The fact that the distance between the peaks in the doublet and septet are the same (710.1 Hz) suggests that these nuclei are coupled to each other.
This characteristic splitting pattern is typical for [PF6]−, a commonly used anion in electrolytes. This demonstrates how NMR spectroscopy can be used to reconstruct the structure of even unknown species.
The information richness of the NMR technique and its ability to be located in the lab, next to where new electrolyte formulations are being developed, can significantly reduce development time and costs. This enables users to confirm their new formulations quickly and efficiently.
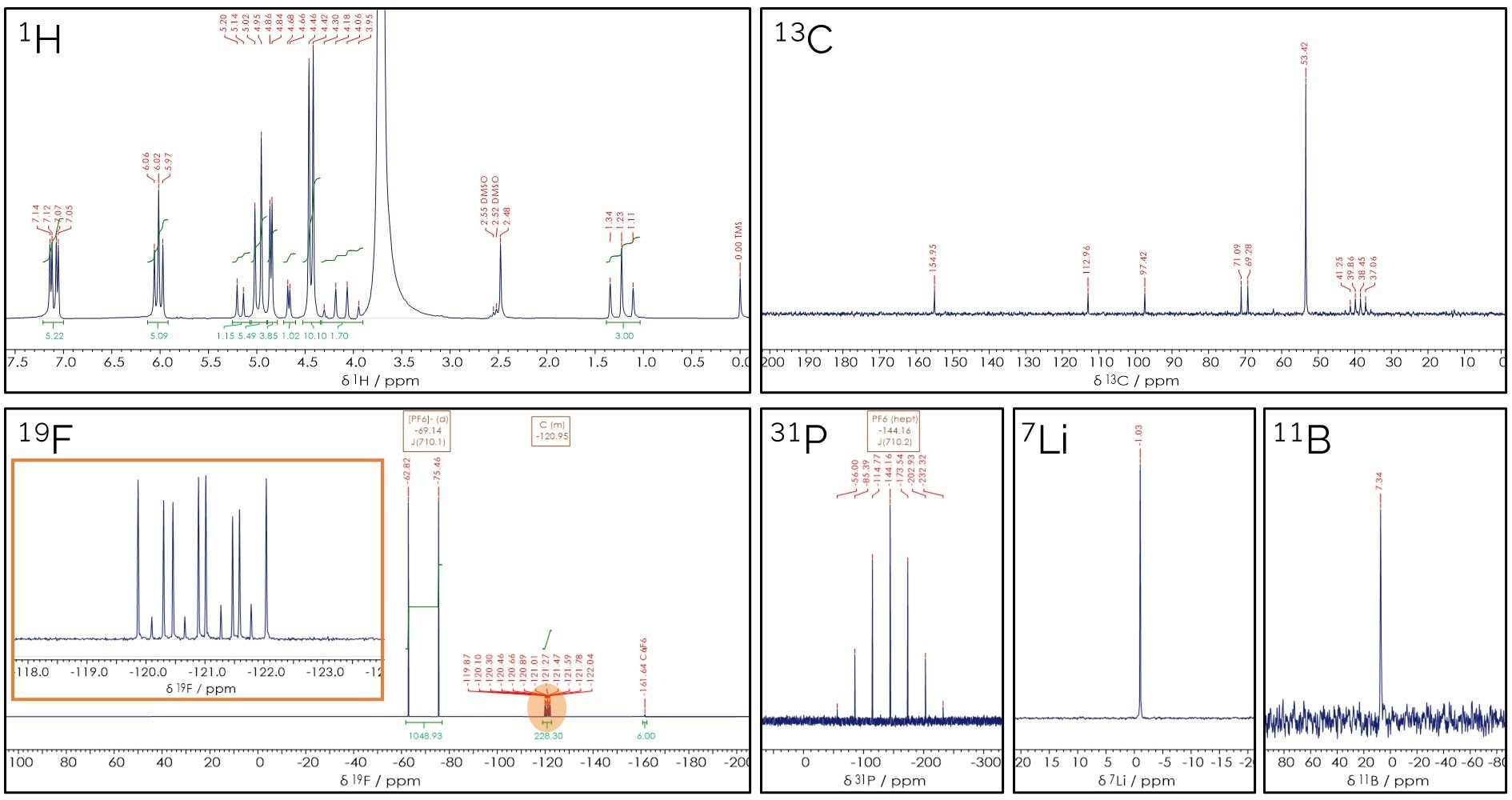
Figure 4. Multinuclear NMR spectra of the same electrolyte taken on a single X-Pulse spectrometer with broadband channel. Image Credit: Oxford Instruments Magnetic Resonance
Quality Control and Reaction Monitoring to Maximize Safety and Lifespan
The X-Pulse's high sensitivity and accessibility make it uniquely suited for quick reaction monitoring and quality control. Its broadband channel allows for the detection of compounds that may not be visible in routine 1H and 13C spectra.
In a case study, two electrolyte solutions with supposedly identical constituents exhibited significant differences in performance when used to build a test cell. Despite no visible differences, questions arose regarding whether it was possible to test electrolyte performance before investing time in building a cell.
The 1H spectrum of the samples, shown in Figure 5, appear identical, offering no insights into the root cause of the issue with one of the mixtures, suggesting that the organic solvent mixture is not the likely cause.
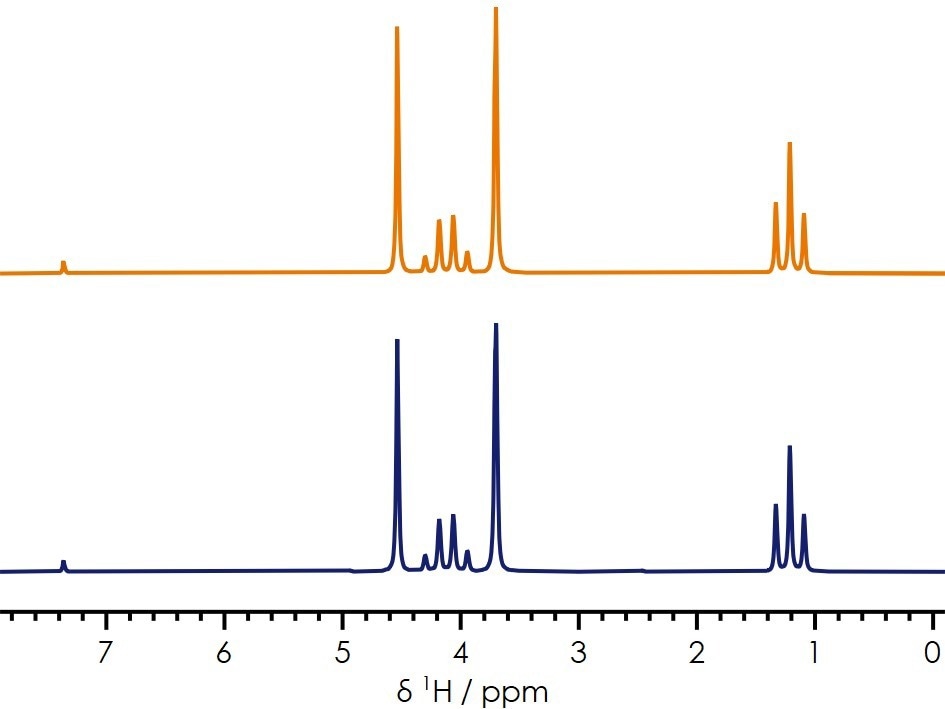
Figure 5. 1H benchtop NMR spectra of two electrolytes presenting different performance characteristics. Image Credit: Oxford Instruments Magnetic Resonance
On the other hand, a 19F NMR spectrum reveals an extra doublet in one of the spectra, which corresponds to a decomposition product of [PF6]− (as shown in Figure 6).
Based on its chemical shift, the decomposition product can be attributed to Difluorophosphoric acid, OPF2(OH), which is a typical byproduct of [PF6]− in the presence of water.
Another species seen in the spectrum is LiF which is a byproduct of the hydrolysis reaction of [PF6]− to OPF(OH)2 and confirms the electrolyte failure has been caused by a hydrolysis reaction in the presence of unwanted water.
Moreover, the X-Pulse is capable of accurately measuring impurities below a concentration of 1% in the electrolyte. It can perform spectrum acquisition with carbon decoupling, thereby eliminating the carbon satellites that may overlap or obscure low-concentration impurities or additives, as demonstrated in Figure 7.
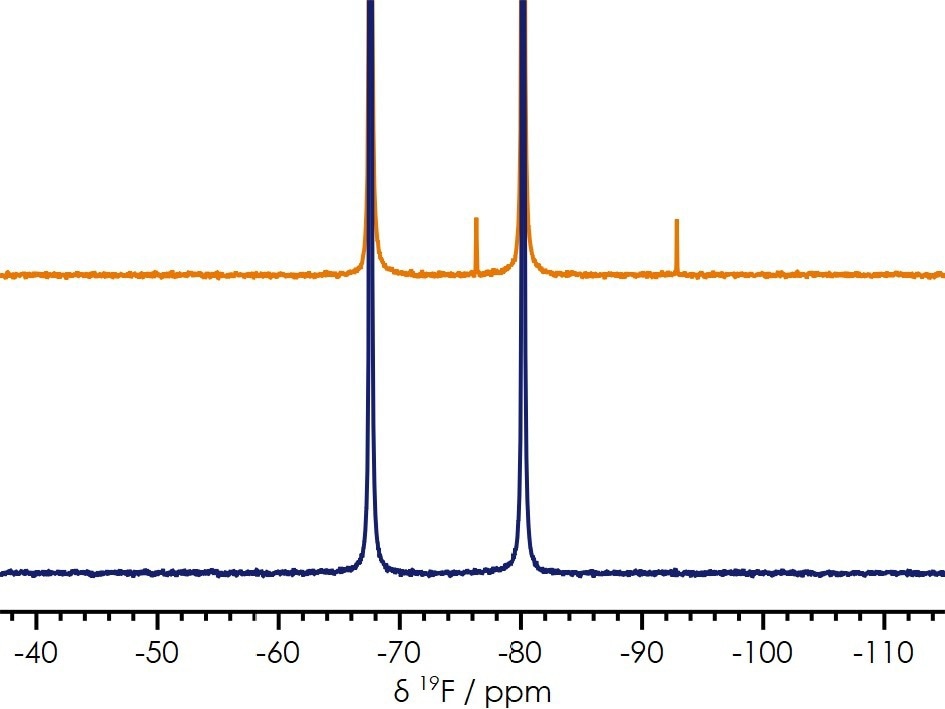
Figure 6. 19F benchtop NMR spectra of two electrolytes presenting different performance characteristics. Image Credit: Oxford Instruments Magnetic Resonance
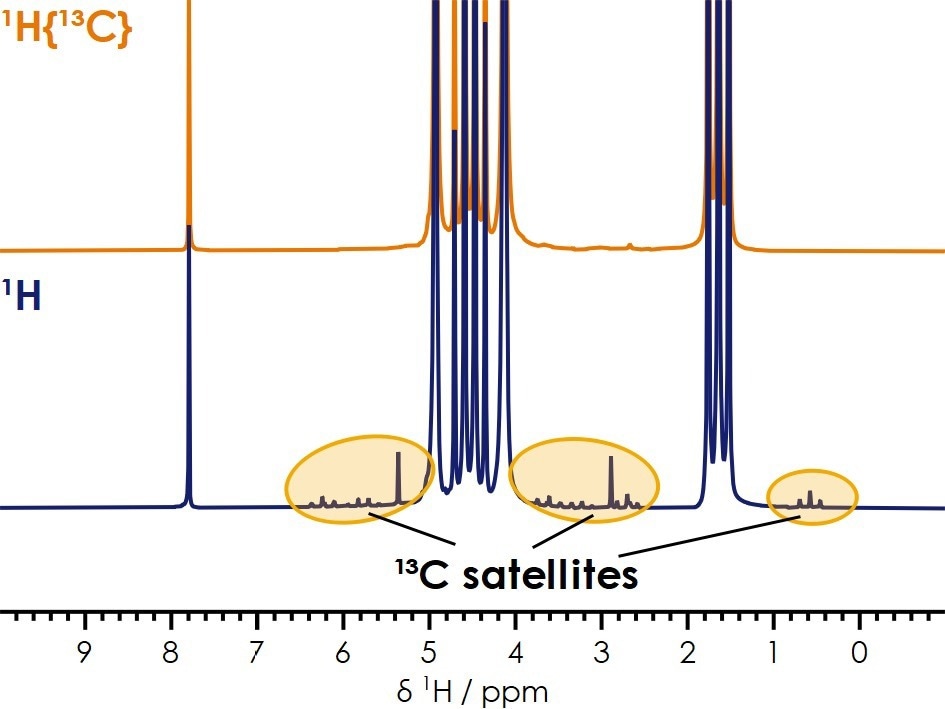
Figure 7. 1H benchtop NMR spectra of the same sample with (top) and without (bottom) 13C decoupling. Image Credit: Oxford Instruments Magnetic Resonance
Beyond just identifying and quantifying degradation and impurities, the form factor of benchtop NMR means that users can continue to take measurements as their formulations age, are exposed to the typical lab environment or are cycled in test cells.
The comprehension of the mechanisms and kinetics responsible for the deterioration of the electrolyte can be facilitated by investigating various factors such as hydrolysis, electrode interaction, exposure to high voltages, and temperature variations, as mentioned earlier.
By comprehending these processes, new combinations of electrolytes and additives can be formulated to mitigate parasitic reactions and considerably enhance cell lifespan.
The process of following the hydrolysis reaction and understanding its kinetics can be illustrated in Figure 8, which integrates the peak areas of 19F spectra collected periodically. This is achieved by exposing a mixture of Li[PF6] and DMC to a small amount of H2O.
![19F peak integrals for different compounds collected every 30 minutes after exposing Li[PF6] in DMC to H2O.](https://d12oja0ew7x0i8.cloudfront.net/images/Article_Images/ImageForArticle_22556_16796327787268270.jpg)
Figure 8. 19F peak integrals for different compounds collected every 30 minutes after exposing Li[PF6] in DMC to H2O. Image Credit: Oxford Instruments Magnetic Resonance
Using Diffusion to Understand Power Density and Performance
The X-Pulse is equipped with pulsed field gradients that allow for experiments beyond mere identification and quantification of materials. By utilizing the Pulsed Field Gradient Spin Echo (PGSE) experiment, diffusion coefficients for individual species in a solution can be determined.
The X-Pulse's broadband capabilities allow for the investigation of even inorganic compounds. This is illustrated in Figure 9, which displays experiments conducted on Li[PF6] in DMC.
A collection of spectra with increasing field gradient strength results in a decrease in the signal strength associated with the diffusion coefficient of the molecules.
The obtained diffusion coefficient value can also provide crucial information for electrolytes, such as their ionic conductivity or cation transference number. Through this experimental feedback loop, the physical properties of electrolytes can be optimized by targeting the most impactful components individually.
The X-Pulse offers variable temperature options that enable the determination of all of these parameters across the majority of the cell's operating temperature range.
This allows for experiments to validate predictions made based on models before constructing test cells, which can greatly expedite the formulation optimization process.
![PGSE series for the determination of the diffusion coefficient of Li[PF6] in DMC.](https://d12oja0ew7x0i8.cloudfront.net/images/Article_Images/ImageForArticle_22556_16796327944211125.jpg)
Figure 9. PGSE series for the determination of the diffusion coefficient of Li[PF6] in DMC. Image Credit: Oxford Instruments Magnetic Resonance
Summary
With its distinctive broadband NMR capabilities, the X-Pulse is capable of providing precise analysis for all compounds frequently utilized in electrolytes, regardless of the workspace. Moreover, this analysis can be conducted over the range of standard operating temperatures for batteries.

Image Credit: Oxford Instruments Magnetic Resonance
Therefore, the performance of electrolytes can be optimized under realistic conditions. The measurements that can be conducted by the X-Pulse support all five of the critical performance parameters discussed within this article:
- Energy Density
- Ion concentration (Li+, Na+)
- Power Density
- Ionic Conductivity and Cation transference over a range of temperatures
- Safety
- Raw material quality
- Electrolyte quality and consistency
- Additive development
- Lifespan
- Understanding of electrolyte degradation
- Additive development
- Cost
- Rapid, low-cost analysis of new formulations
- Reduced cost of poor quality
This makes benchtop NMR a perfect fit to support this industry which will be crucial to ensuring the future sustainability of our planet.

This information has been sourced, reviewed and adapted from materials provided by Oxford Instruments Magnetic Resonance.
For more information on this source, please visit Oxford Instruments Magnetic Resonance.