Poly- and perfluoroalkyl substances (PFAS) detection and quantification have gained significant attention within analytical chemistry.1 This field has become very important because of the association of these "forever chemicals" with substantial health risks prompting the implementation of stricter regulations.1

Image Credit: IONICON Analytik
In the past, most analytical investigations relating to PFAS were carried out offline, employing time-intensive methodologies such as gas chromatography (GC) and liquid chromatography (LC).2 Although these methods are considered highly reliable for targeted sampling, they do not facilitate real-time monitoring of compounds.
This study presents initial findings from a comprehensive series of real-time direct-injection PTR-MS investigations on PFAS. The study includes an examination of ion chemistry, experimentally determined limits-of-detection (LoDs), and a proof-of-concept indoor air study.
For the experiments, an off-the-shelf PTR-TOF 6000 instrument with a mass resolution of approximately 6000 m/Δm and a sensitivity of around 2000 cps/ppbv was employed.
Notably, the IONICON PTR-TOFMS instruments, including the one used in this feasibility study, feature TRU-E/N® ion chemistry, eliminating the need for compound-specific calibration during quantification.
Perfluorotributylamine (PFTBA)
PFTBA (C12F27N) has been identified as the most potent greenhouse gas discovered to date. It is obtainable as a gas standard with a certified concentration of 980 pptv in an N2 matrix. The gas standard was dynamically diluted to determine the limits of detection (LoDs).
Through dissociative PTR reactions of H3O+ with PFTBA, a product ion at m/z 413.977 (C8NF16+) is formed.
The PTR-TOF 6000 demonstrates remarkable sensitivity, complemented by the extensive transmission range of IONICON's hexapole ion guides and the minimal chemical background in this high mass region, resulting in outstanding LoDs:
- 2.4 pptv after only one second of integration time
- 280 ppqv with a one-minute integration time
When employing NO+ and NH4+ (produced using the patented ammonia-free method)3 as reagent ions, no product ions associated with PFTBA are observable. However, with O2+ reagent ions, reactive dissociative charge transfer occurs, generating product ions at m/z 501.971 (C9NF20+) and m/z 263.987 (C5NF10+).
Perfluorooctanol (FTOH)
A singular petri dish moistened with FTOH (C8H5F13O) was employed to establish an initial indoor air concentration of 6.7 ppbv within an office room measuring approximately 30 m³ (LxWxH: 410x250x290 cm; RT: 23-26 °C), with two walls predominantly composed of windows and featuring limited ventilation.
The petri dish was removed 8.8 minutes after the start of the experiment. Subsequently, the indoor air quality was meticulously monitored for 48 hours, utilizing an extended integration time (initially set at one second and later increased to 30 seconds).
At the 20-minute mark into the experiment, the measured concentration declined to 50%, followed by a further decrease to 10% of the original 6.7 ppbv after approximately five hours.
Intriguingly, after a lapse of 24 hours from the experiment's commencement, the direct sunlight exposure elevated the room's surface temperature, causing an instantaneous concentration surge by a factor of 2.3.
Briefly opening the windows to ventilate the room after 48 hours resulted in a temporary decrease in FTOH concentration, with 85 pptv remaining after 63 hours.
This value still stands at five times the already heightened (as a result of previous experiments) background level of 17 pptv recorded at the commencement of the experiment.
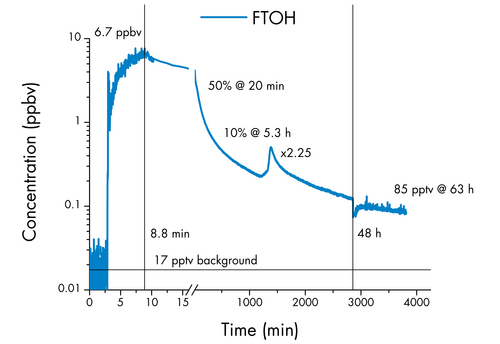
Image Credit: IONICON Analytik
Perfluorobutanoic Acid (PFBA)
Simultaneously with the release of FTOH, PFBA (C4HF7O2) was also introduced into the indoor air through a wetted petri dish.
PFBA holds particular significance due to its tendency to accumulate in the lungs, with higher plasma levels being associated with a more severe prognosis of COVID-19.4
However, in contrast to FTOH, the concentration of PFBA in the indoor air swiftly decreased, reaching only 50% of its initial level of 676 pptv within a mere 10 minutes and further dropping to 10% after 25 minutes.
As the experiment progressed for two hours, the concentration dwindled to below 10 pptv and remained stagnant even as the sun heated the room.
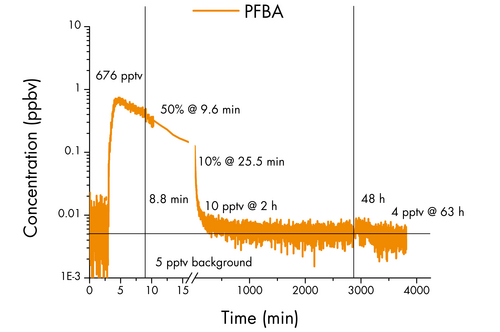
Image Credit: IONICON Analytik
Conclusion
The three presented examples showcase the exceptional performance of IONICON's PTR-TOF 6000 in effectively quantifying PFAS in real-time within the air. Moreover, an extensive investigation covering a broader range of PFAS compounds will soon be published.
In rare instances where the performance of the PTR-TOF 6000 may still prove insufficient, the newly introduced FUSION PTR-TOF 10k is highly recommended. This instrument offers 20-40 times higher sensitivity and approximately twice the mass resolution.
The Next-Gen PTR-TOF has the innovative Fast-SRI ion source, allowing almost instantaneous reagent ion switching. It also features the ultra-clean FUSION reaction chamber, ensuring detection limits below 200 ppqv. Notably, this instrument holds TRU-E/N certification.
References and Further Reading
- echa.europa.eu/hot-topics/perfluoroalkyl-chemicals-pfas, European Chemicals Agency (17.11.2022).
- S. F. Nakayama et al., Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment. TrAC Trends in Analytical Chemistry 2019, 121, 115410.
- US11342171 (B2), CN111386590 (B), EP3503161 (B1).
- P. Grandjean et al., Severity of COVID-19 at elevated exposure to perfluorinated alkylates. PLoS ONE 2020, 15(12).

This information has been sourced, reviewed and adapted from materials provided by IONICON Analytik.
For more information on this source, please visit IONICON Analytik.