Sponsored by Xenocs SASReviewed by Louis CastelJul 17 2023
Poloxamers are a class of synthetic triblock copolymers that consist of blocks of polypropylene oxide (PPO) and polyethylene oxide (PEO). They are also known under commercial names as Synperonics®, Kolliphor®, or Pluronics®.
Poloxamers are of significant interest since they are valuable in various applications due to their unique properties.1
The amphiphilic nature of Pluronics enables them to self-assemble in aqueous solutions, forming core-shell micelles. This property is particularly valuable in drug delivery systems, where Pluronics may be employed to encapsulate hydrophobic drugs to make them more soluble and bioavailable.
Pluronics can also undergo reversible gel-sol transitions in response to changes in concentration and temperature. They are instrumental in wound healing, tissue engineering, and drug delivery.2
Since the rheological behavior of Pluronics can be finely tuned by adjusting the temperature, concentration, and other parameters, there has been research into using Pluronics as additives to control fluid rheology, including thickeners or viscosifiers in personal care products.3 Their extremely low toxicity means they are safe for pharmaceutical and personal care products.
Pluronics have found applications in microfluidics and materials science outside of medical sciences. For example, they can be utilized in digital microfluidics or the production of nanoplates in various applications such as electronics and catalysis.
In an aqueous solution, these block copolymers can form micelles at or above a specific concentration, known as the critical micelle concentration (CMC).4 Various factors, including concentration, temperature, pH, and the size of the PPO block, impact the CMC.
The properties of the micelles can be determined by adjusting the concentration and composition of the Pluronics. For example, spherical micelles form at moderate temperatures and low concentrations.
As the temperature increases, rod–like micelles begin to form because of packing constraints imposed by their size increase.
Under certain conditions, the micelles pack together through the entanglement of the PEO chains to produce cubic and hexagonal phases. The phase diagram presented in Figure 1 demonstrates the limits in terms of temperature and concentration of the available phases formed by Pluronic P123.
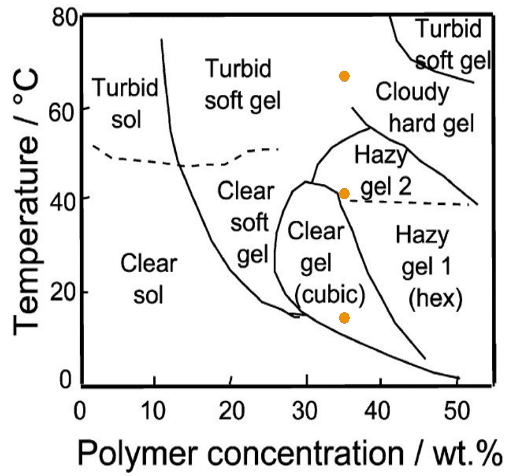
Figure 1. Phase diagram of Pluronic P123 obtained from tube inversion flow tests and visual inspection, together with structural assignments based on scattering experiments. The orange circles indicate the measuring positions of this application note. Credit: J. of Coll. Int. Sci., 2009. DOI: 10.1016/j.jcis.2008.09.054. Image Credit: Xenocs
The below application note discusses the structural evolution as a function of temperature being monitored for the P123 at 35 wt%. Multiple phases may be attained by adjusting the temperature at this concentration.
Click here to gain access to the complete application note
The Xenocs Couette stage mounted on a Xeuss 3.0 HR was employed for the brief shearing of the sample, followed by a SAXS measurement to probe the relationship between the structures formed and the rheological behavior.
References and Further Reading
- G. E. Newby, I. W. Hamley, S. M. King, C. M. Martin, and N. J. Terrill, Structure, Rheology and Shear Alignment of Pluronic Block Copolymer Mixtures, Journal of Colloid and Interface Science 329, 54 (2009). DOI: 10.1016/j.jcis.2008.09.054
- A. Torcello-Gómez, M. Wulff-Pérez, M. J. Gálvez-Ruiz, A. Martín-Rodríguez, M. Cabrerizo-Vílchez, et al., Block Copolymers at Interfaces: Interactions with Physiological Media, Advances in Colloid and Interface Science 206, 414 (2014). DOI: 10.1016/j.cis.2013.10.027
- S. R. Bhatia, A. Mourchid, and M. Joanicot, Block Copolymer Assembly to Control Fluid Rheology, Current Opinion in Colloid & Interface Science 6, 471 (2001). DOI: 10.1016/S1359-0294(01)00122-4
- P. Alexandridis and T. Alan Hatton, Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) Block Copolymer Surfactants in Aqueous Solutions and at Interfaces: Thermodynamics, Structure, Dynamics, and Modeling, Colloids and Surfaces A: Physicochemical and Engineering Aspects 96, 1 (1995). DOI: 10.1016/0927-7757(94)03028-X

This information has been sourced, reviewed and adapted from materials provided by Xenocs.
For more information on this source, please visit Xenocs.