Users often feel a sense of accomplishment when achieving well-defined, isolated spots with a thin-layer chromatography (TLC) plate. Users set up a flash chromatography method, prepare the column, load the sample, and eagerly await the elution of the separated samples.
However, the peaks may begin to rise steadily, reach the apex, descend, and then unexpectedly ascend again. This frustration is a common sentiment shared by many who attempt to translate a method from TLC to flash chromatography.
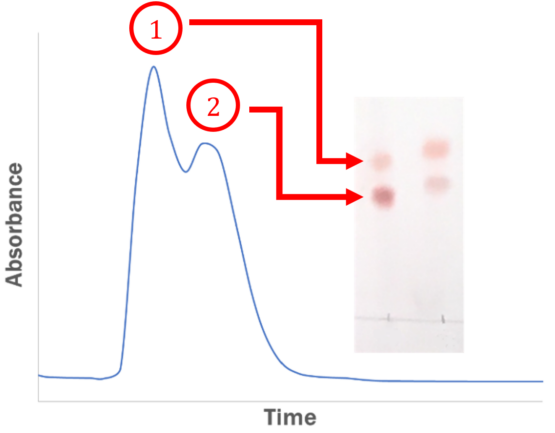
Image Credit: Sorbent Technologies, Inc.
This article will investigate the technical reasoning behind the inability to reproduce TLC using flash chromatography.
Across the board, TLC plates typically utilize granular silica gel with particle sizes ranging from 5-15 µm, whereas flash chromatography primarily uses granular silica gel ranging from 40-63 μm.
Why Are They Different?
The fundamental driving force for solvent movement in TLC is capillary. Smaller particles facilitate better capillarity and faster solvent movement. In flash chromatography, the solvent movement relies on pressure. The smaller the particle, the higher the pressure required for efficient solvent flow through the column.
In both TLC and flash chromatography, smaller particles enhance separation efficiency. However, sample diffusion is increased with the larger particles, which can lead to peak broadening. This phenomenon significantly impacts the desired separation, most notably overlapping peaks, as illustrated above.
The number of theoretical plates or the separating power of the silica can also influence considerable differences in the desired separation. These factors also contribute to peak narrowing, which creates a more efficient separation between two or more samples.
Significance of Sample Loading
Another crucial factor scientists often do not consider is the difference between loading samples on a TLC plate and loading samples onto a flash column.
The TLC plate’s separation may suffer if the sample is still wet when development starts, similar to loading a liquid sample onto a flash column. Dry loading onto a column improves resolution, as is typically the case with a TLC plate where the sample is usually dry.
Why not use 5-15 µm Silica Gel in a Flash Column?
Smaller silica gel particles (5 µm or smaller) are used with many HPLC columns, offering significantly higher resolution and separation efficiency. However, the substantially higher pressure requires the need for a steel column. Plastic (polypropylene) columns cannot withstand the higher pressures associated with particles smaller than 20 microns.
Steel columns are costly and non-disposable, making smaller particles prohibitive for flash column separation.
Instead of employing 40-63 µm silica gel for flash chromatography, an alternative approach is using silica gel with particle sizes ranging from 20-45 µm. This choice improves the separation efficiency and resembles a TLC-like separation more closely while maintaining a lower pressure system and allowing easier packing.
While reproducing the separation achieved by a TLC plate may be challenging, it is possible to come close by modifying the size of the silica and by dry loading the sample onto the column.
Acknowledgments
Produced from materials originally authored by Robert Cotta.

This information has been sourced, reviewed and adapted from materials provided by Sorbent Technologies, Inc.
For more information on this source, please visit Sorbent Technologies, Inc.