Weight per unit volume, known as density, is a crucial physical property for coating materials, as well as other liquids and semiliquids. It serves as an initial indicator of the composition ratios within a substance. Many materials are crafted and manufactured by weight but marketed based on volume.
Determining the density of nonviscous liquids is typically straightforward, involving measuring a standard volume of the material.
These nonviscous substances do not trap air and allow air to rise and escape at the surface. Conversely, in the case of viscous materials, the derived density may be inaccurate as they can trap air, leading to misleading volume readings.
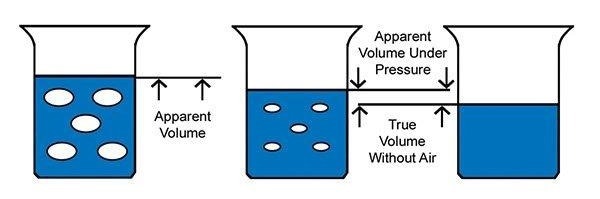
Image Credit: Paul N. Gardner Company, Inc.
The image displays the false apparent volume of a viscous material with entrapped air, represented by the blue liquid. Applying pressure to the material reduces the apparent volume due to the compression of entrapped air. The genuine volume of the material excludes the entrapped air.
For nonviscous materials, determining density in pounds per gallon is easily achieved using a standard "weight per gallon cup" and a scale capable of reading to the nearest tenth of a gram.
A widely accepted cup accommodates a weight of distilled water at 77 °F, amounting to 83.2 g. Converting the gram weight to pounds per gallon is a simple process—divide by 10 (move the decimal one place to the left), given that the absolute density of water at 77 °F is 8.32 lb./gal.
Diluting and Mixing
Accurate volume determination for viscous materials with entrapped air involves dilution and mixing with an acceptable diluent (thinner). This process frees the viscous material sample from entrapped air.
The diluted and mixed materials are then filled into a standard weight-per-gallon cup and weighed. The illustrated images outline the procedure for determining the density of viscous materials with entrapped air.
Combining diluent (A) with the viscous material sample (W) effectively releases trapped air. The weight-per-gallon cup (B) then holds a level-full mixture of the diluent and viscous material.
Determining the genuine weight per gallon of the viscous material, devoid of entrapped air, involves the application of the formula detailed below.
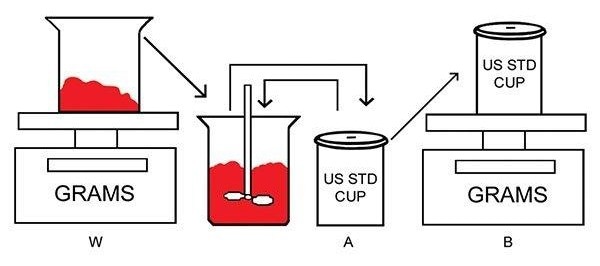
Image Credit: Paul N. Gardner Company, Inc.
X= (W x B) / (W + A - (10 x B))
Where,
- X: the unknown lb/gal density of the viscous material.
- W: the measured gram weight of the viscous material sample.
- A: the gram weight of the diluent from a full weight-per-gallon cup.
- 10: the multiplier to convert "B" into the gram weight of a filled weight-per-gallon cup of the viscous material.
Proof of the formula is provided as follows:
lb/gal = (grams x lb/gal) / (grams + grams - (K x lb/gal))
lb/gal = grams/10. This is the cup conversion factor.
Substituting the conversion factor in the original formula:
grams/10 = (grams x grams/10) / (grams + grams - K x grams/10)
K is the conversion factor equal to 10.
Substituting 10 for K:
grams/10 = (grams + grams/10) / (grams + grams - grams)
grams/10 = grams/10
1 = 1
This method underwent laboratory confirmation using two materials with significantly different densities. Varying the unknown material (W) quantity from 25 grams to 125 grams consistently yielded a calculated weight per gallon (X) within 0.5% of the known true value.
The weight-per-gallon cups are available in corrosion-resistant materials. One such cup features a sidewall of series 316 stainless steel, a machined bottom, and a closely fitted lid of series 416 stainless steel. A radiused area where the bottom attaches to the sidewall ensures easy cleaning.
The lid is designed with a small opening to facilitate the discharge of excess material, ensuring the precise specified volume of the cup contents. These cups come with a warranty to be within 0.5% of the specified volume. Some cups qualify for volume certification under MIL-STD 45662, surpassing the requirements of ASTM methods.
Cups of various volumes are available, including a "mini" cup, one-tenth the U.S. standard cup. Another cup easily converts pounds and imperial gallons. These cups can also be utilized in this indirect computational measurement method, provided the correct multiplier is applied in the equation for "B."
Copied with permission from the March 1989 issue of Industrial Finishing Magazine Copyright© 1989, Hitchcock Publishing Co. All rights reserved.

This information has been sourced, reviewed and adapted from materials provided by Paul N. Gardner Company, Inc.
For more information on this source, please visit Paul N. Gardner Company, Inc.