X-Ray photoelectron spectroscopy (XPS) is one of the most important and widely used methods for determining surface chemistry of materials. Cryo-XPS, where the sample is cooled to temperatures below −150 °C, increases the range of samples that can successfully be analysed.
Although XPS has been widely used for the characterization of many types of materials for over five decades, its application to biological systems is still relatively restricted. This limitation is primarily due to the stringent vacuum conditions that are required for XPS.
Biological samples are typically hydrated, which is incompatible with ultra-high vacuum. One strategy for XPS analysis of these samples involves freezing the liquid by maintaining cryogenic temperatures throughout the pump-down and analysis processes. The cryogenic sample handling system available for the AXIS Supra+ XPS instrument uses liquid nitrogen to cool sample temperatures below −150 °C.
XPS Analysis of Biological Samples
Removing water from a biological system by spraying or freeze-drying to allow XPS analysis may seem practical. However, dehydration of such samples may result in the collapse of the cell structure or the introduction of surface contaminants. Therefore, a better approach is XPS analysis of hydrated samples without the drying step. This can be achieved via one of two approaches.
Near-ambient pressure XPS (NAP-XPS) allows the analysis of samples in a gaseous water vapor environment in the mbar pressure range. This approach requires a complex, dedicated NAP-XPS spectrometer. A simpler and more universally applicable approach is the use of cryo-XPS. This capability can be incorporated into a conventional high-performance X-ray photoelectron spectrometer.
Ramstedt and Shchukarev have demonstrated successful cryo-XPS in several publications.1-3 Using a fast-freezing technique, they have demonstrated that after pumping to ultra-high vacuum, a few layers of water still cover the solid material,4 in addition to water inside the sample structure. For biological samples, this relates to structural water at the surface as well as inside cells, enabling analysis of intact cells without cell collapse.
Biological samples, by their nature, are complex. Due to the surface sensitivity of XPS and the physical dimensions of a bacterial cell envelope, only the outermost structures are analyzed. As with most analytical techniques, careful interpretation of the data is required to relate it back to the material being analyzed.
Specific methodologies have been developed to deduce the substance composition concerning the bacterial cell envelope's three main organic building blocks. Namely, lipids/hydrocarbon-like compounds, polysaccharides, and proteins/peptides.
Reducing X-Ray Sample Damage
Although XPS is often cited as a non-destructive technique, X-ray irradiation can cause damage to sensitive samples. For some samples, visible discoloration may be observed.
However, changes in surface chemistry induced by the X-rays or high kinetic energy secondary electrons may only be visible as changes in the acquired spectra. Sample damage is easily assessed by ratioing a wide survey spectrum taken at the start and end of an XPS experiment.
Importantly, cooling the sample with liquid nitrogen during analysis can minimize these changes in chemistry. The value of cryo-XPS has proven for the analysis of lithium-sulfur battery (LSB) materials. Detailed analysis of high-resolution S 2p spectra (figure 1) for uncycled and uncharged LSB materials provides valuable insights into surface chemistry and its evolution over time.
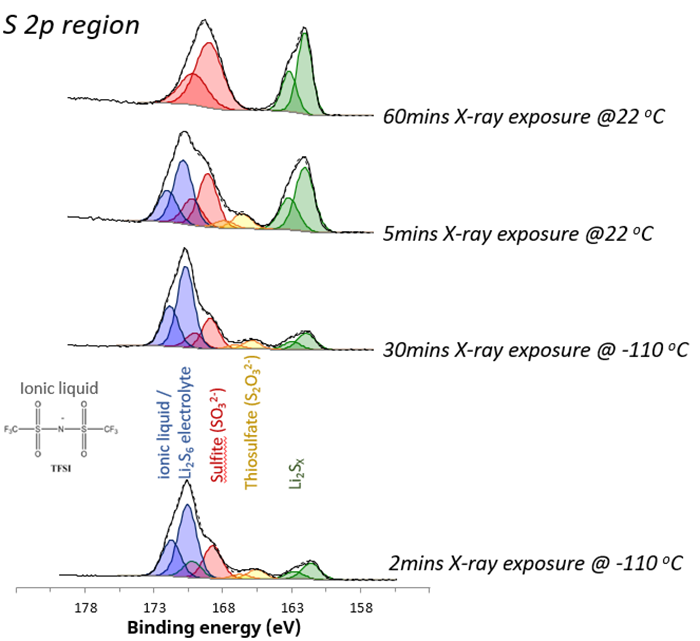
Figure 1. S 2p chemical states acquired under different analysis conditions. Image Credit: Kratos Analytical, Ltd.
For this material, when the surface is cooled, it exhibits stability under X-rays. Over 30 minutes, the relative composition of the different chemical states remains largely unchanged, with a minimal change of less than 5 %. At room temperature, stability diminishes, and decomposition and radiolysis processes are initiated.
High-order sulfur species (blue) are reduced to lower-order species, particularly evident in the significant increase in lithium sulfide components (green).
The ionic liquid on the surface recombines, forming surface sulfates. This recombination process is marked by a nearly three-fold increase in surface lithium concentration, indicating the formation of surface sulfides.
Similarly, the carbon chemistry of the sample is stabilized by in-situ cryo-XPS analysis (figure 2). Changes were observed during room temperature analysis, where a change from CF3 to CFx chemical state underscored the dynamic nature of the material’s composition and chemistry.
This example demonstrates the importance of cryo-XPS for analyzing battery systems and other materials prone to degradation under X-ray irradiation.
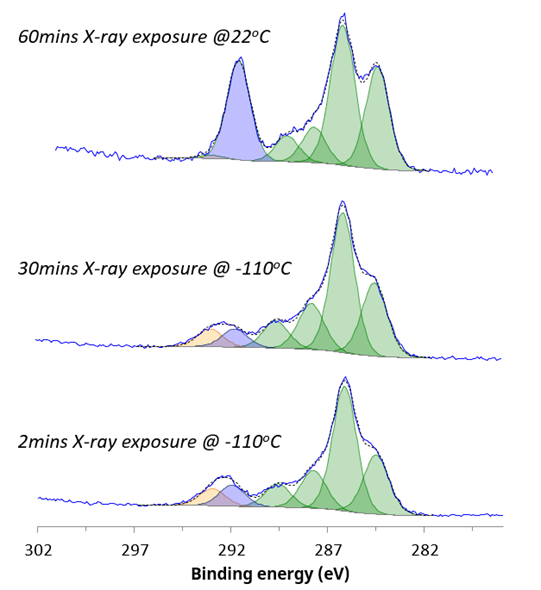
Figure 2. C 1s chemical states acquired under different analysis conditions. Image Credit: Kratos Analytical, Ltd.
High Vapor Pressure Sample Analysis
As with hydrated biological samples, outlined above, XPS analysis of samples with high vapor pressure can only be achieved in combination with liquid nitrogen cooling. The molecule 1,4-dibromobenzene has been studied as a precursor for the synthesis of conjugated polymers with applications in nano-electronic and electronic devices.
The vapor pressure of 1,4-dibromobenzene means that it will volatilize in a vacuum at room temperature. Cooling of the 1,4-dibromobenzene ensured that it remained in its solid, crystalline form throughout introduction to the spectrometer and during XPS measurements. The survey spectrum (figure 3) shows that in addition to bromine and carbon, there is < 1.5 atomic % contribution from oxygen.
Analysis of the narrow region scans allowed detailed interpretation of the chemical state of the molecule and identification of bulk material as well as under-coordinated bromine at the solid-vacuum interface. These results are discussed in greater detail elsewhere.5
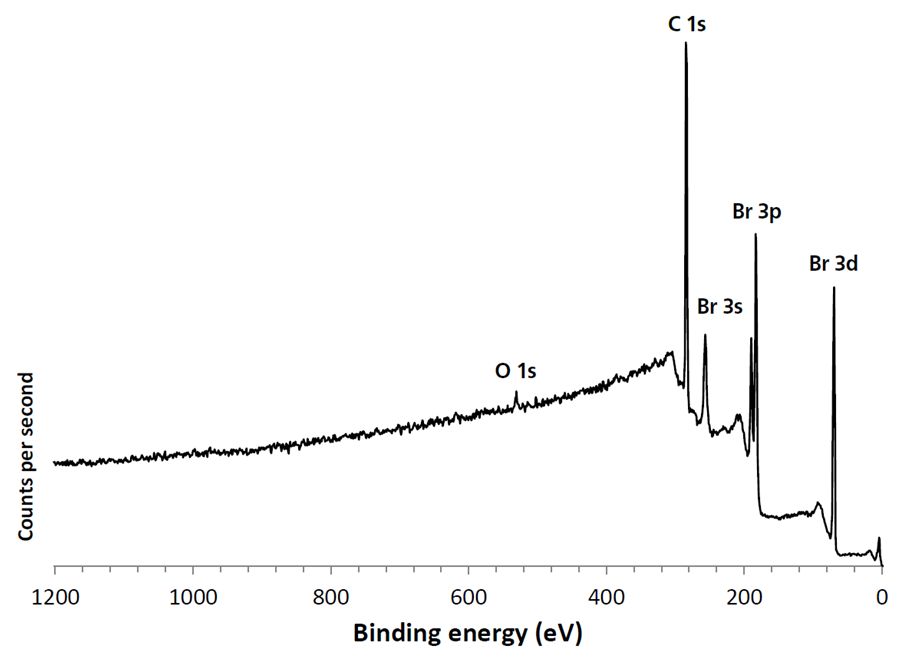
Figure 3. Large area cryo-XPS survey spectrum of high vapor pressure 1,4-dibromobenzene. Image Credit: Kratos Analytical, Ltd.
Low-Cost, High Value
The ability to perform cryo-XPS experiments on a range of samples as well as minimize X-ray-induced sample damage offers a significant advantage over standard room-temperature XPS analysis.
Cryo-XPS capability can be configured on an AXIS spectrometer when purchasing the main instrument, whilst upgrading existing instrumentation is also possible. Sample temperature is regulated by a PID controller with an optional computer interface for control through supplied software.
As demonstrated by the examples above, the inclusion of the relatively low-cost cryo-XPS option can add high value by allowing a significantly greater range of samples to be analyzed.
References
- Shchukarev, A., Gojkovic, Z., Funk, C., and Ramstedt, M. (2020). Cryo-XPS Analysis Reveals Surface Composition of Microalgae. Appl. Surf. Sci. 526, 146538. doi:10.1016/j.apsusc.2020.146538
- Shchukarev, A., and Ramstedt, M. (2017). Cryo-XPS: Probing Intact Interfaces in Nature and Life. Surf. Interf. Anal. 49, 349–356. doi:10.1002/sia.6025
- Shchukarev, A., Rosenqvist, J., and Sjöberg, S. (2004). XPS Study of the Silica-Water Interface. J. Electron. Spectros. Relat. Phenomena 137 (140), 171–176. doi:10.1016/j.elspec.2004.02.095
- Shchukarev, A., Boily, J. F., and Felmy, A. R. (2007). XPS of Fast-Frozen Hematite Colloids in NaCl Aqueous Solutions: I. Evidence for the Formation of Multiple Layers of Hydrated Sodium and Chloride Ions Induced by the {001} Basal Plane. J. Phys. Chem. C 111, 18307–18316. doi:10.1021/jp075321c
- https://www.kratos.com/application-areas/application-downloads/xps-analysis-frozen-14-dibromobenzene (last accessed 11/01/2024)

This information has been sourced, reviewed and adapted from materials provided by Kratos Analytical, Ltd.
For more information on this source, please visit Kratos Analytical, Ltd.