Sponsored by ProtoReviewed by Maria OsipovaFeb 27 2024
Physiochemical properties such as solubility and dissolution rate play a crucial role when it comes to the therapeutic efficacy of oral drug formulations. According to the Biopharmaceutical Classification System, approximately 90% of new drug candidates belong to either class II (60-70%) or class IV (10-20%), which indicates modest water solubility and low intestinal permeability.1
Such a combination results in poor drug absorption and insufficient concentrations of the drug in the body, meaning that it cannot reach its maximum therapeutic potential.
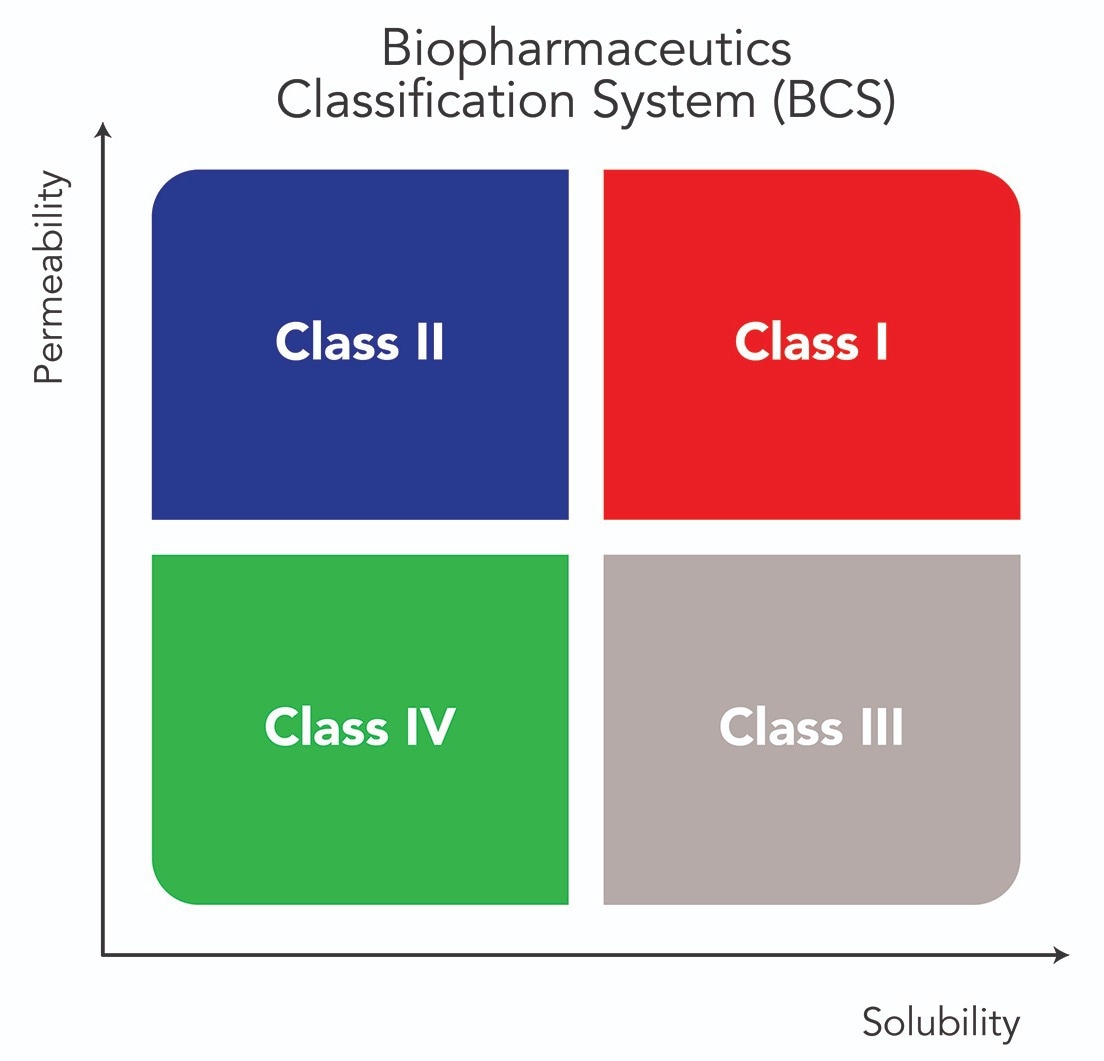
Image Credit: Proto
Physiochemical properties can be modified on the atomic level by altering the drug’s molecular structure (e.g., introducing hydrophilic functional groups to facilitate solubility in water); however, any change to the structure of the drug may lead to undesirable therapeutic effects or adverse side effects.2
Crystal engineering is an approach that allows scientists to tune the physical properties of pharmaceutical materials in the solid form through deliberate control of intermolecular interactions between the molecules of the active pharmaceutical ingredient (API) while still retaining their original chemical functionality. This manipulation of APIs is often accomplished through the design and formation of polymorphs, salts, hydrates, solvates, and co-crystals.

Prof. Nicholas Vukotic and Dr. Anton Dmitrienko examine a 3D-printed crystal structure at the University of Windsor as part of their crystal engineering research. Image Credit: Proto
For example, valsartan and sacubitril are used to regulate blood pressure. Co-crystallization of both drugs in the presence of sodium cations results in a new crystalline phase (patented as Entresto® by Novartis) with 50% higher bioavailability of valsartan than the valsartan administered alone.3
Many crystal engineering methods are scalable and simple to implement, making them attractive to the pharmaceutical industry. These methods include solid-state grinding, solution-reaction crystallization, solvent evaporation, slurry conversion, and hot-melt extrusion.4 Reaction conditions can be controlled and optimized to attain the desired purity of the final material. Given the number of experimental variables at play, the discovery of new drug formulations and optimization of experimental conditions for their synthesis are usually done in a high-throughput fashion.
High-throughput powder X-ray diffraction (HT-PXRD) allows for the characterization of up to 1024 samples with a single well plate in a matter of hours. A refined micro-X-ray beam moves across the wells continuously, probing various regions of the sample (see figure below).
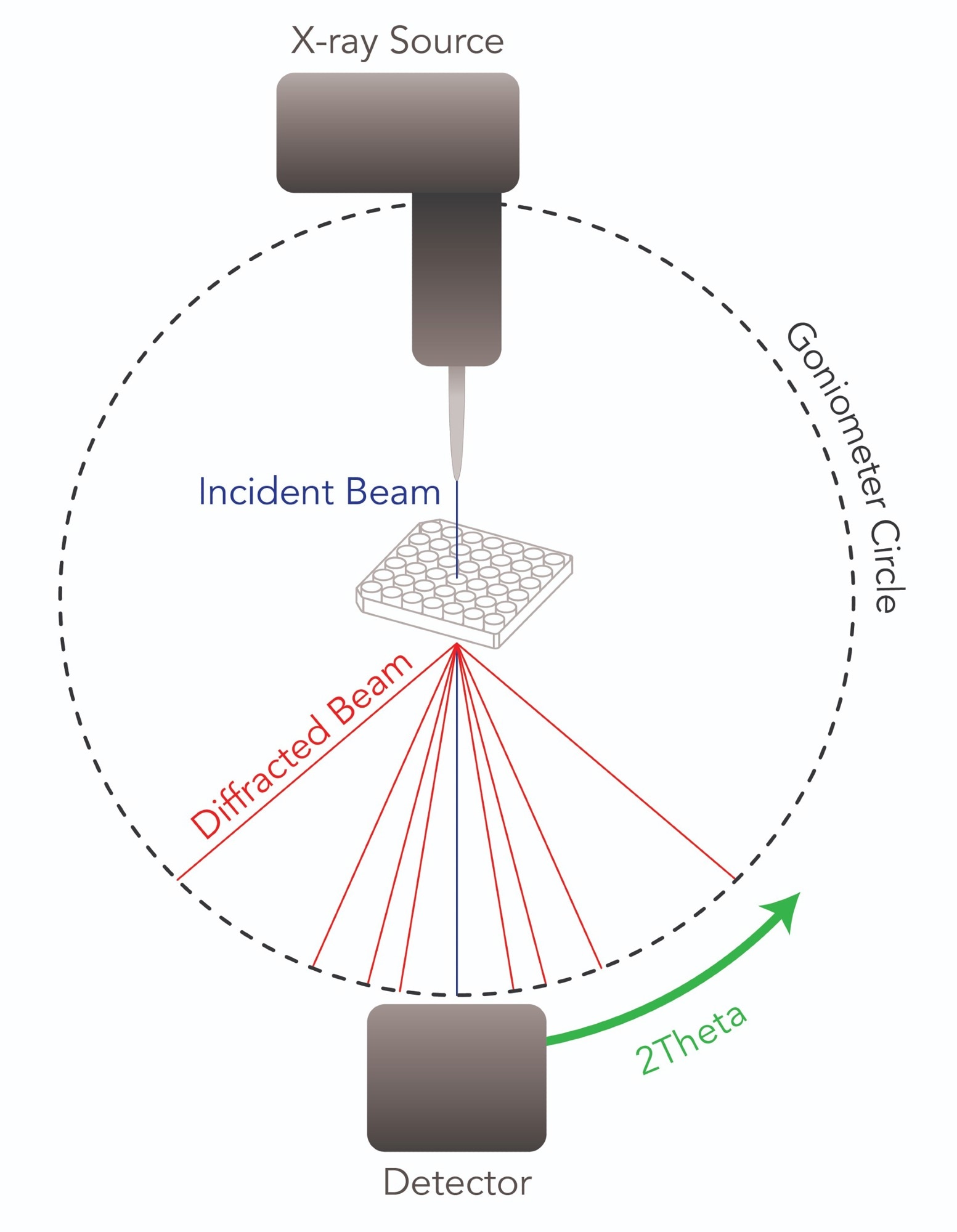
Schematic illustration of the diffraction geometry in a transmission experiment with an area detector. Image Credit: Proto
Crystalline powder samples diffract X-rays in accordance with Bragg’s law, forming diffraction peaks that are recorded by a detector installed on a goniometer.
Diffraction peaks (and the resulting diffraction patterns they form) contain information about the crystal structures present in the sample. By comparing measured diffraction patterns with database entries, one can identify the presence of unreacted initial reagents, formed by-products, and new phases that have yet to be reported in the database. Given the distinctiveness of diffraction patterns, all observed peaks from the collected data should match with the peaks from the database entry to confirm the presence of a particular phase. This is why this analytical technique is called fingerprinting.
Consider the following PXRD pattern. A thorough search across entries in the COD and CCDC database revealed the presence of two phases identified as sulfanilic acid and zinc oxide.
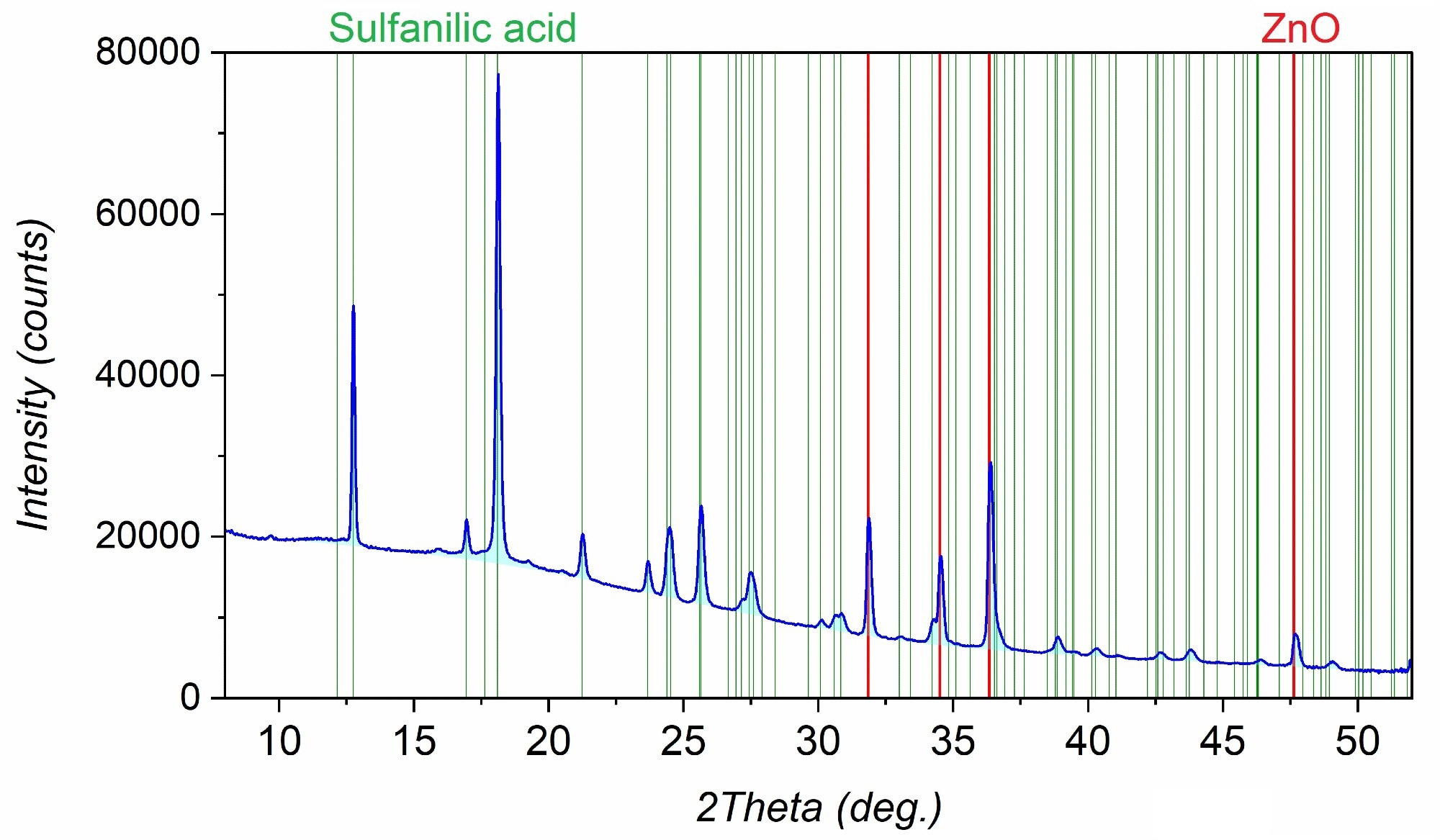
PXRD pattern of the sample represented by a mixture of sulfanilic acid and zinc oxide. Image Credit: Proto
Cluster analysis can be employed to expedite analysis of the large number of diffraction patterns collected during high-throughput screening. This method treats every diffraction pattern as an array of data points. Comparing all datasets point by point, the algorithm can reveal similar patterns and group them together. Identifying common traits in the group allows one to extrapolate the results to all samples within the same group. Thus, the analysis of a large array of data may only require analysis of a few groups.
The following dendrogram was plotted for 22 samples obtained after mechanochemical optimization of a co-crystal formation. Every sample was prepared by using the same API and the same co-former; however, the solvent used for the synthesis was different for each sample.
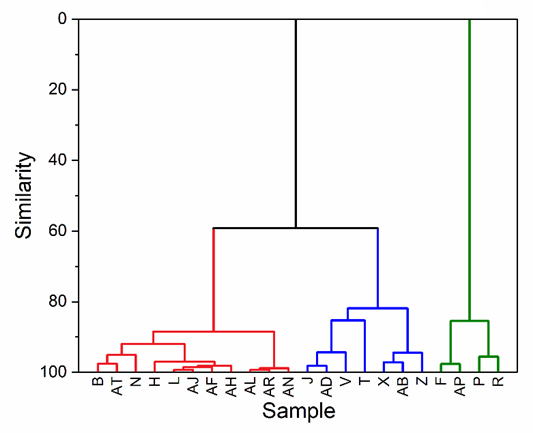
Dendrogram showing the separation of 22 PXRD patterns into three distinctive groups based on similarity. Image Credit: Proto
Cluster analysis shows three distinctive groups of patterns (red, blue, and green). While the red and blue groups belong to the same clade (or branch), the green group stands out. Further phase analysis confirmed the presence of a new co-crystal phase in the green group, while the samples identified as the red and blue groups were a mixture of initial reactants.
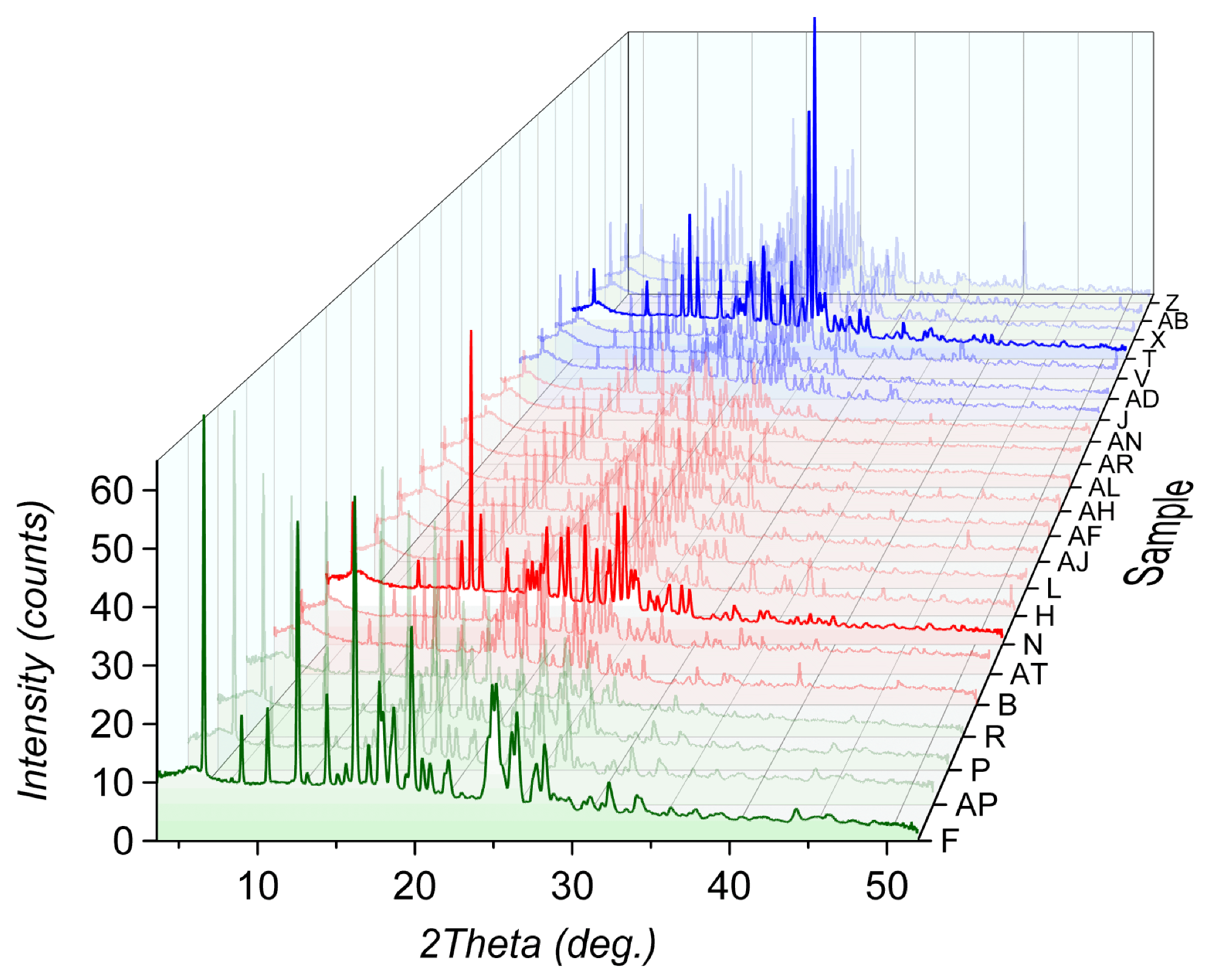
3D waterfall-PXRD overlay of samples in red, blue, and green groups. Image Credit: Proto
When considering an HT-PXRD system for a pharmaceutical lab, scientists should be aware of some key elements that will help ensure optimal results.
Transmission geometry
While working with light-absorbing samples (organic small molecules and proteins), transmission geometry maximizes diffraction signal by letting the X-ray beam travel through the bulk of the sample, dramatically increasing the sample-illumination volume.
Copper anode
The use of a copper-anode X-ray tube generates an X-ray wavelength of 1.54 Å. The longer wavelength of copper (compared to other common anode materials like Mo or Ag) is less penetrative and, therefore, better interacts with light scatterers, increasing the diffraction signal's intensity.
Additionally, the wavelength of X-ray radiation is proportional to the peak separation of the diffraction pattern. Longer wavelength results in better peak separation, which facilitates the analysis of molecular samples in which the number of peaks is especially high (due to low symmetry).
Microfocus and X-ray optics
A microfocus X-ray source can increase the brightness of the incident beam more than tenfold compared to traditional sealed X-ray tubes. Microfocus X-ray tubes enable the use of Montel optics, which further refine the beam, eliminating the contribution of the Kβ X-ray component and focusing the beam at the surface of the detector to minimize peak broadening.
Hybrid photon-counting detectors
Detectors that utilize hybrid photon-counting technology are known to provide low background and high efficiency of signal capture. Large area detectors can simultaneously collect intensities from a wide range of 2theta angles.
Well plate technology
Large well plates enable the characterization of many samples without user intervention. Proto’s AXRD LPD-HT is equipped with a motorized XYZ stage and comes with a well plate holder that is compatible with commercially available well plates used in the pharmaceutical and biochemical industry, allowing for the collection of up to 1024 samples with just one well plate.
Extended functionality
Additional in-situ stages, such as variable temperature/pressure units or humidity chambers, enable expanded testing of the stability of new drug formulations.
The Proto AXRD LPD-HT system in a well plate configuration. Image Credit: Proto
References
- Bhalani, D. V. et al. Biomedicines. 2022, 10, 2055.
- Thomas, G. Fundamentals of Medicinal Chemistry, 1st ed.; Wiley-Blackwell, 2003.
- Bandaru, R. K. et al. Front. Pharmacol. 2021, 12, 780582.
- Guo, M. et al. Acta Pharm. Sinica B. 2021, 11, 2537.

This information has been sourced, reviewed and adapted from materials provided by Proto.
For more information on this source, please visit Proto.