One of the greatest hurdles to overcome when developing new battery materials with high energy density is capacity degradation with cycling. Various causes of capacity degradation include particle cracking, lithium retention in electrodes, electrolyte degradation, and dendrite formation.

Image Credit: sommart sombutwanitkul/Shutterstock.com
A complete understanding of these degradation mechanisms is a key step toward successfully identifying new battery materials.
X-Ray diffraction (XRD) can facilitate the evaluation of failure mechanisms in-situ by analyzing basic changes in the crystal structure during battery cycling.
Malvern Panalytical’s Empyrean XRD platform provides several options for in-situ cycling different types of battery cells, from coin cells and electrochemical cells to pouch cells and prismatic cells.
Coin and Electrochemical Cells: Opening Possibilities for Non-Ambient Exploration
The Empyrean XRD can assess all coin cell types with an X-Ray transparent window on at least one side. Malvern Panalytical offers a specialized coin cell holder for use during charge-discharge cycling.
Another option is the electrochemical cell, with an X-Ray transparent window of beryllium or glassy carbon. Malvern Panalytical can supply electrochemical cells with heating and cooling option when mounted on the Empyrean XRD.
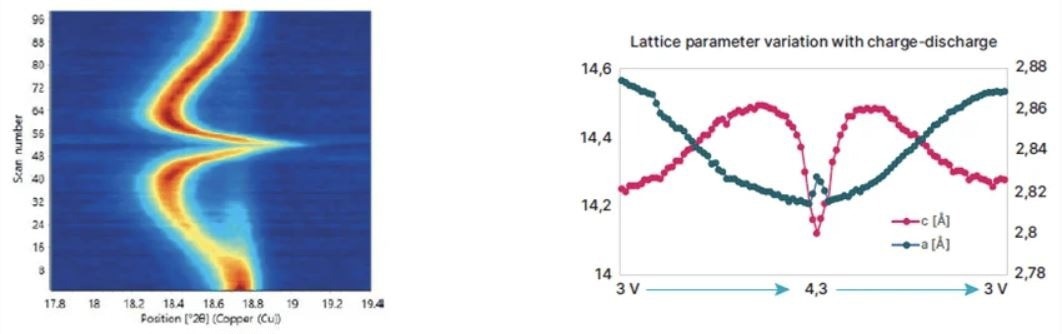
Example of in operando cycling of an NCM cathode and graphite anode in an electrochemical cell. The image on the left shows how the 003 peak shifts during cycling. The right image shows how c and a lattice parameters change during charge and discharge. Any abrupt change in lattice parameter is usually associated with crystal phase changes and may cause particle cracking. Image Credit: Malvern Panalytical Ltd

Image Credit: Malvern Panalytical Ltd

VTEC: Variable Temperature Electrochemical cell for in-situ (-10 to 70 ºC) in-operando XRD measurements. Image Credit: Malvern Panalytical Ltd
Pouch and Prismatic Cells: Unlocking the Full Story with Hard Radiation Transmission
The Empyrean XRD facilitates the analysis of multilayer pouch cells up to 5 mm thick when the platform is equipped with high-energy Ag radiation and a GaliPIX3D detector.
The Empyrean enables 60 kV excitation, allowing for the high-intensity, 22.16 keV Ag radiation necessary for pouch cell research. Unique multilayer focusing mirrors provide high resolutions coupled with a high-brightness X-Ray beam, further reducing the measurement times.
Measurements were conducted using the Empyrean XRD platform armed with Ag Kα radiation and the GaliPIX3D detector. Each XRD scan over the 5-30 2θ range was measured in just five minutes. In total, 166 scans were measured over five complete charge-discharge cycles.
The voltage variation (3.2V in the discharged state to 4.3V in the fully charged state) is plotted and shown via the green color line. The isoline plot exhibits cathode and anode peak positions when the cell is charged or discharged. The peak around 6.8° 2θ is the 003 peak of the NCM cathode, and its varying position demonstrates changes in the c-parameter with cycling. The discontinuous peak around 9° 2θ comes from the graphite anode, which switches from C to LiC6 or LiC12 throughout the charge cycle and then reverses back to LiC12 and then C during the course of the discharge cycle.
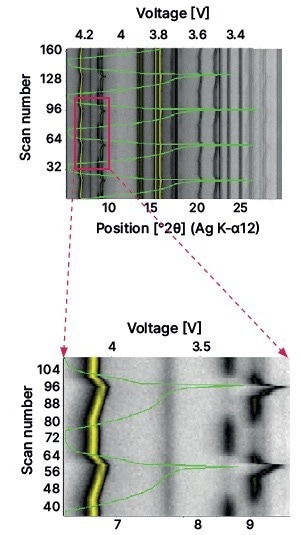
Image Credit: Malvern Panalytical Ltd

Pouch and prismatic cell mounting on the Empyrean XRD. A mechanism to apply pressure on the pouch cell is also supported. Image Credit: Malvern Panalytical Ltd
VTEC-Trans: for non ambient (-10 to 70 ºC) in-operando measurements on pouch cells. Image Credit: Malvern Panalytical Ltd

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical Ltd.
For more information on this source, please visit Malvern Panalytical Ltd.