Photothermal AFM-IR, commonly referred to as AFM-IR, is an analytical technique used to understand the chemistry of a material at the nanoscale. It combines the nanoscale spatial resolution of atomic force microscopy with the chemical analysis capabilities of infrared spectroscopy.
In contrast to alternative nanoscale spectroscopic techniques, including scattering scanning nearfield optical microscopy (s-SNOM) and tip-enhanced Raman spectroscopy (TERS), AFM-IR offers a range of significant advantages. It is simple to use and easy to interpret, and measurement speeds and data repeatability are fast and reliable.
Typically, it only takes a few seconds to run an AFM-IR spectrum. This results in the rapid accumulation of point spectra and optimized collection times for hyperspectral imaging. The AFM-IR technique can capture sub-10 nm spatial resolution while demonstrating monolayer sensitivity at various probing depths.
However, various experimental factors can play a role in influencing IR band intensities, positions, and shapes, and should be considered when interpreting AFM-IR spectra. This article outlines what photothermal AFM-IR can offer and the experimental factors influencing spectral interpretation.
What is Photothermal AFM-IR?
In isolation, atomic force microscopy is typically used for high-resolution imaging of varying samples to acquire topographic, mechanical, electrical, and other properties.
This method achieves a spatial resolution of less than 10 nm by using a sharp tip, and in some cases, it can resolve down to the molecular or even atomic scale. On the other hand, infrared spectroscopy is a powerful optical technique for precise chemical identification, as each material has unique absorption bands in the "fingerprint" region due to its specific molecular vibrations.
When these two techniques are applied simultaneously, AFM-IR can perform chemical identification with a spatial resolution several orders of magnitude below the optical diffraction limit, which usually has a value of several to multiple tens of microns for a mid-infrared source.
AFM-IR is easy to operate in comparison to alternative complementary nanoscale infrared spectroscopy techniques, most notably scattering scanning near field optical microscopy (s-SNOM) and tip-enhanced Raman spectroscopy (TERS). Furthermore, the AFM-IR spectra correlate with FTIR spectra.
AFM-IR measures the cantilever oscillation signal, which is directly proportional to the infrared absorption by the sample. In contrast, the long-standing technique used to measure the complex optical properties of samples, s-SNOM, requires complicated interferometric detection systems and relies on theoretical modeling. This typically leads to peak shifts in spectra and inconsistent band shapes and peak ratios in relation to the FTIR spectrum.1
Raman spectroscopy is a powerful and widely accepted analytical technique for molecular identification and structural analysis. However, the Raman scattering process has a very low cross-section, especially for vibrations involving light elements, and heavily relies on the manufacturing of field-enhancing tips for TERS. This dependence results in limited consistency and reproducibility. TERS acquired from protein molecules are also generally lacking where amide bands are concerned, which restricts their use for identifying protein secondary structures.2
AFM-IR sets itself apart from s-SNOM and TERS due to its close correlation with transmission FTIR spectra relative to peak shapes, positions, band ratios, and high signal-to-noise ratios.
History of the AFM-IR Technique
The AFM-IR technique was first developed by Alexandre Dazzi using a “bottom-up” configuration, and commercial instrumentation based on this technique (nanoIR™), which was first developed in 2010 by Anasys Instruments and later acquired by Bruker in 2018.3
Significant advances in AFM-IR capabilities have been made in recent years; these are demonstrated in terms of sensitivity, spatial and spectral resolutions, measurement time, and fields of application.
Later generations of nanoIR instruments (nanoIR2™, nanoIR3™) adopted a “top-down” configuration, which does not require an IR-transparent substrate and greatly improves the variety of samples that can be studied.
Based on Bruker’s industry-leading Dimension Icon® AFM platform, the Dimension IconIR™ system is the most advanced, highest-performance AFM-IR instrument in the world today. This is due to the low noise and high stability of its AFM, high sensitivity and numerous modes of AFM-IR measurement, and availability of relevant correlative studies of mechanical, electrical, and thermal properties.
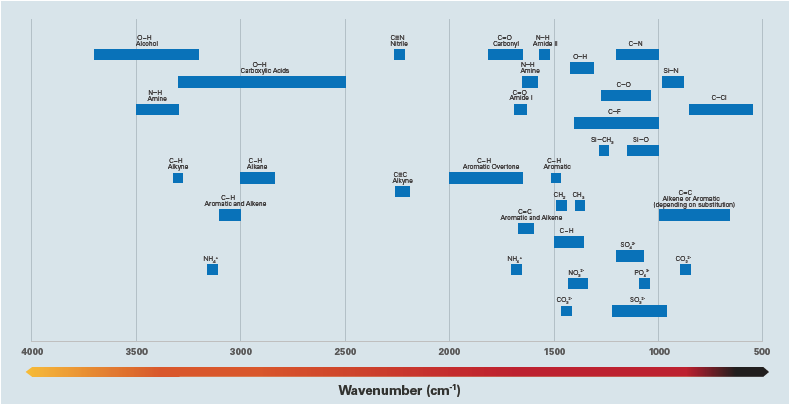
Figure 1. Typical IR absorption frequencies for common functional groups. Image Credit: Bruker Nano Surfaces and Metrology
Working Principles
AFM-IR involves using an AFM tip to detect a sample’s local thermal expansion, which is determined by its absorption of infrared radiation at the tip location. Consequently, this results in a spatial resolution on the scale of the AFM tip radius. To capture AFM-IR measurements, a pulsed infrared laser beam is concentrated onto a region of the sample in proximity to the AFM tip.
When the infrared beam is absorbed, the sample undergoes instantaneous thermal expansion, generating a transient force that causes the AFM cantilever to oscillate. In standard AFM-IR mode, this oscillation can occur across multiple eigenmodes. Alternatively, a single eigenmode can be selectively enhanced in Resonance-Enhanced or Tapping AFM-IR modes.
AFM-IR spectra are acquired by measuring the AFM cantilever oscillation amplitude as a function of wavelength while preserving the AFM tip at fixed xy positions on the sample. AFM-IR maps illustrating how chemical species are distributed across a sample are generated by scanning the sample while illuminating it with fixed wavelengths.
Hyperspectral imaging has become more popular with the improvement of measurement speeds. It allows for the complete measurement of the AFM-IR spectra on a dense grid of points, which provides detailed information about molecular species and their distributions in the sample. In experimental conditions, a pulsed laser source, together with optics for beam steering, focusing, and polarization control, is required for good AFM-IR performance.
To ensure the laser stays aligned with the tip, a sample xy scanner is required. Even with a small temperature increase (<10 K with OPOs; <1 K with QCLs) and a standard thermal expansion coefficient (10-6 to 10-4), the eventual photothermal expansion is on the scale of several to tens of picometers for a polymer sample with a thickness of hundreds of nanometers.
The sample's rapid thermal expansion affects the cantilever's instantaneous equilibrium position, converting thermal expansion on the scale of tens of picometers into nanometer-scale cantilever oscillations. With advancements in sensitivity, the AFM-IR technique can now detect a single monolayer sample exhibiting significantly lower thermal expansion.
The physics of how AFM-IR works is relatively basic and well-researched. The overall AFM-IR signal intensity as a result of the infrared absorption by the sample at the tip location is expressed in the equation below:4,5
| SAFM-IR ∝ HAFM Hexp Iinc (λ) α(λ) |
EQUATION 1. |
Where HAFM and Hexp are contributed via the AFM cantilever oscillation dynamics and the sample thermal expansion coefficient, Iinc (λ) is the laser power at the tip, and α(λ) = 4πκ(λ)/λ is the sample absorption coefficient shown at the wavelength λ with κ(λ) as the hypothetical part of sample refractive index.
From this equation, while the intensity of the AFM-IR signal depends on other material properties of the sample, such as the thermal expansion coefficient and mechanical stiffness, these properties remain constant at a specific point on the sample. Therefore, they do not affect the relative peak intensities or peak shapes.
Therefore, the AFM-IR signal measured as the oscillation amplitude of the cantilever at a fixed point (X0, Y0) on a sample is equivalent to the absorption coefficient:
| SAFM-IR (X0, Y0) ∝ α(λ, X0, Y0) |
EQUATION 2. |
Moreover, the AFM-IR spectrum relates directly to the bulk FTIR spectra obtained in transmission mode. This creates a basis for applying AFM-IR as a nanoscale chemical identification technique, as the AFM-IR spectra can be cross-referenced digitally against commercial databases of FTIR spectra and otherwise identified like any conventional transmission FTIR spectrum.
AFM-IR spectra correspond with transmission FTIR spectra for a diverse range of materials. Below is a comparison of an AFM-IR spectrum extracted from a 300 nm-thick polystyrene film to an FTIR transmission spectrum.
The spectra show matches in the CH-stretching region (2800-3200 cm-1) and the fingerprint region (1000-1800 cm-1).6,7 This emphasizes the power of AFM-IR spectroscopy as a chemical identification technique on the nanometer spatial scale, which is beyond the capabilities of traditional FTIR instrumentation.
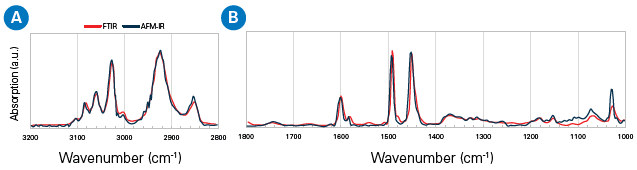
Figure 2. Comparison of AFM-IR and bulk FTIR spectra of a polystyrene film: (a) CH-stretching region, (b) fingerprint region. Image Credit: Bruker Nano Surfaces and Metrology
Major Operational Modes
Since the AFM-IR technique was first developed, Bruker and its collaborators have developed several AFM-IR modes, including Resonance-Enhanced mode, Tapping AFM-IR mode, Surface Sensitive mode, and PeakForce Tapping AFM-IR mode. Each mode has exhibited exceptional strengths and considerably extended AFM-IR capabilities, enabling significant breakthroughs in several research fields.
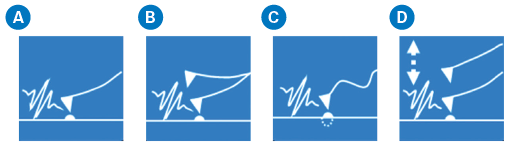
Figure 3. Major AFM-IR modes: (a) contact or Resonance-Enhanced mode, (b) Tapping AFM-IR mode, (c) Surface Sensitive mode, and (d) PeakForce Tapping AFM-IR mode. Image Credit: Bruker Nano Analytics
When selecting the right mode for AFM-IR measurement, the sample properties and the goal of the measurement should be considered. An outline of the AFM-IR modes, the laser source, sensitivity, spatial resolution, and likely applications are shown in Table 1.
Table 1. Major AFM-IR modes implemented on the nanoIR/IconIR systems. Source: Bruker Nano Surfaces and Metrology
| Mode |
Laser
source |
Sensitivity |
Spatial
resolution (nm) |
Applications |
| Conventional |
Ekspla
OPO |
>50 nm |
>50 |
Polymeric and biological material with thickness >50 nm |
| Resonance-Enhanced |
Daylight QCL, M Squared Firefly; APE Carmina |
monolayer |
>20 |
Monolayer, single molecule |
Tapping
AFM-IR |
Daylight QCL, M Squared Firefly; APE Carmina |
monolayer |
<10 |
Loose particle, soft/sticky material |
| Surface Sensitive |
Daylight QCL,M Squared Firefly; APE Carmina |
monolayer |
<10 |
Multilayer, thin coating on bulk |
Traditional AFM-IR Mode
AFM-IR is typically run in contact mode, with simple signal acquisition and low demands on the light source while still offering the primary advantage of correlation to transmission FTIR.
After the sample absorbs radiation, the ringdown of the AFM cantilever oscillations is analyzed using the fast Fourier transform (FFT) technique to determine their amplitudes and frequencies. As a result, local absorption spectra can be generated by measuring the cantilever ringdown's peak-to-peak intensity or the cantilever oscillation's amplitude at a selected frequency as a function of the source wavelength.
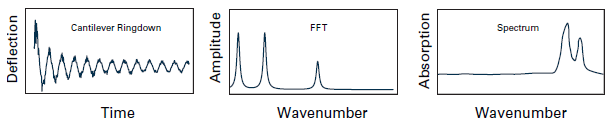
Figure 4. Diagrams of the cantilever ringdown, FFT, and spectrum for conventional AFM-IR operation. Image Credit: Bruker Nano Surfaces and Metrology
The conventional AFM-IR mode typically requires a sample thickness of more than 50 nm to acquire a detectable AFM-IR signal. Collecting an AFM-IR spectrum with a range of 1000 cm-1 takes 2-4 minutes, while the laser wavelength is slowly tuned incrementally.
Resonance-Enhanced AFM-IR Mode
Bruker’s patented Resonance-Enhanced mode was an important development and breakthrough that brought about key improvements in AFM-IR sensitivity that utilizes innovative developments in Quantum Cascade Laser (QCL) technology.8 Usually, the mid-infrared laser source is either a QCL or M Squared Firefly laser, both with a tunable pulse repetition rate. The AFM cantilever is set to contact mode as it engages with the sample, while the laser pulse repetition rate is tuned to correspond with the contact resonance frequency of the cantilever.
Thermal expansion is caused by how the sample absorbs infrared radiation, which results in uninterrupted excitation of the cantilever. This differs from conventional AFM-IR, which typically demonstrates decay in the ringdown. Any contact resonance frequency can be selected for AFM-IR measurements.
Typically, when running AFM-IR spectroscopy, the second cantilever resonance frequency near 180 kHz is selected due to its good signal-to-noise ratio. A Resonance-Enhanced AFM-IR spectrum is acquired by plotting the amplitude of a selected cantilever resonance in the frequency domain as a function of the laser wavelength. For AFM-IR imaging, a higher resonance frequency (>1 MHz) may be selected, as it offers greater spatial resolution because of the reduced thermal diffusion length between two adjacent laser pulses.
The Resonance-Enhanced mode considerably boosts AFM-IR sensitivity due to the significantly enhanced AFM cantilever oscillation. The AFM-IR signal boost or gain from resonant excitation is Q/(2π), where Q represents the quality factor of the cantilever mode, expressed as the resonance amplitude over its full width at half maximum.
The signal amplification is typically 5 to 40 times in relation to the ringdown mode. This facilitates AFM-IR measurements to be acquired on thin samples, such as a self-assembled monolayer with thickness as little as <1 nm on a silicon substrate.
The exceptional sensitivity of this mode has allowed measurements of infrared absorption spectra and chemical maps for a single protein molecule, with the results enabling the true determination of the secondary structure of a single protein molecule that is in accordance with the structure of the bulk protein material.9
In contrast to the traditional AFM-IR mode, the spatial resolution of Resonance-Enhanced mode shows improvements and can achieve levels as low as 20 nm for relatively smooth samples.
The speed of Resonance-Enhanced AFM-IR spectroscopy also shows improvement, as the laser wavelength is typically scanned in a fast sweep mode. A spectrum with a range of 1000 cm-1 can be acquired anywhere between 1–10 seconds. To make up for the mechanical difference between the sample’s different components, a phase-locked loop (PLL) is typically applied to ensure the match between the laser pulse rate and the contact resonance frequency of the cantilever is preserved on the sample.
Tapping AFM-IR Mode
Conducting AFM-IR measurements using a tapping technique marks another important technological progression. Bruker developed its own TappingMode™ to enable high-resolution topography measurements on rough, soft, or adhesive samples, previously deemed impossible or presented significant challenges when set in contact mode. The same can be said of Tapping AFM-IR.10,11
Tapping AFM-IR significantly enhances spatial resolution to below 10 nm, sometimes less than 5 nm. This advancement in spatial resolution makes it possible to acquire practical infrared absorption images on samples with fine structures, such as block copolymers and various semiconductor samples with small domains.
Tapping AFM-IR can achieve high sensitivity when measuring thin monolayer samples. The AFM cantilever is operated in TappingMode, in which the probe is driven by a piezo actuator with intermittent oscillations at one of the cantilever resonance frequencies (f2 or f1) so that the tip periodically contacts (i.e., ‘‘taps’’) the sample. The Tapping AFM-IR signal is detected at an alternative resonance frequency (f1 or f2) and is relative to the absorption coefficient of the sample at the tip location.10
The assembly for the Tapping AFM-IR mode is typically a QCL or Firefly laser paired with different Tapping AFM-IR probes. During operation, the semi-soft TnIR-A probe drives at a frequency close to 60 kHz in the first mode and a detection frequency near 380 kHz in the second mode.
The TnIR-D probe is more rigid and operates with a driving frequency near 1600 kHz in the second mode and is detected in the first mode near 250 kHz. Resultingly, the higher signal-to-noise ratio of the TnIR-D probe makes it the default probe when running Tapping AFM-IR.
Surface Sensitive AFM-IR Mode
Surface Sensitive AFM-IR mode (SSM) is a recently developed Bruker proprietary mode that aims to isolate and only measure the top layer of a sample by limiting signal contributions from sub-surface material.12 While its full capabilities are still being explored, SSM has the potential to measure the top layer of a sample with minimal preparation.
In SSM, a soft AFM cantilever engages with the sample in contact mode. Heterodyne detection is applied when tuning the laser pulse repetition rate to match the sum or difference of two contact resonance frequencies of the cantilever. This mode differs from Tapping AFM-IR, which utilizes two tapping frequencies.
In SSM, the cantilever's drive runs at a high resonance frequency near 2 MHz and is detected at a lower resonance frequency around 180 kHz, with a comparatively high laser pulse repetition rate. This combination of experimental settings offers greater surface sensitivity than Resonance-Enhanced mode or Tapping AFM-IR mode.
For a standard polymer sample, SSM can limit signal contributions to only the top 10–30 nm of the sample.
PeakForce Tapping AFM-IR Mode
The PeakForce Tapping AFM-IR (PFIR) mode, developed by Bruker collaborator Xiaoji Xu and their co-workers, is an innovative and powerful mode of the AFM-IR technique.13
PFIR leverages Bruker’s patented PeakForce Tapping® mode, in which the AFM tip periodically contacts the sample, and the approach and retraction routines drive at several kilohertz. Thanks to smart synchronized averaging and background subtraction mechanisms, PeakForce Tapping can run faster than conventional force curves. This means the advantages of the off-resonant approach, i.e., delivering quantitative nanomechanical data, remain intact.
PFIR takes advantage of the contact time during each PeakForce Tapping cycle to transduce the infrared absorption of the sample, enabling infrared spectroscopy and imaging capacities at sub-10 nm spatial resolution. Through precision control of the interaction force between the AFM probe and the sample, PFIR is well-suited for use on rough and sticky samples.
Since PFIR uses PeakForce Tapping, one key benefit is the capacity to map several key material properties simultaneously, including topographic, chemical, mechanical (e.g., adhesion, modulus), and electrical (e.g., surface potential).
AFM-IR in Liquid Mode
AFM-IR in liquid mode has been developed on the nanoIR system with bottom-up illumination. The AFM-IR in Liquid mode has enabled researchers to resolve the secondary structures of protein samples in water. Results have revealed that there are comparable signal-to-noise and lateral resolutions taken from AFM-IR spectra and maps of samples in water to those in air.14
Previously, it was thought that capturing such measurements would be impossible due to the strong mid-infrared absorption by water. Further work revealed that Tapping AFM-IR functions better than Resonance-Enhanced mode for samples in a fluid environment.
These developments create additional possibilities for AFM-IR analysis of a diverse range of air-sensitive samples. As alternatives to immersion in a liquid phase, preparing selected samples as nanodroplets on a substrate with a nanospray or sealed in a hydrated environment with a thin hydrogel coating is possible.15,16
These new techniques allow samples to be measured using the standard top-down illumination and are more likely to preserve the native structures in liquid. For full insights on experimental accessories and laser sources, see Bruker’s full application note, ‘A Comprehensive Guide to Photothermal AFM-IR Spectroscopy’.
Conclusions
The physics of Photothermal AFM-IR is well understood and has shown to be a good match when compared to transmission FTIR data. This allows researchers to gain key insights from bulk spectroscopy which can be applied directly at the nanoscale.
As outlined in this article, several key factors must be considered when working at the nanoscale for measuring, processing, and characterizing AFM-IR spectra.
By understanding these essential factors, AFM-IR spectroscopy enables sub-diffraction measurements applicable to polymer, biological, semiconductor, and inorganic samples, offering various characterization pathways for materials researchers across various fields.
References
- A C Jones, and M B Raschke, Nano Letters 12, 1475 (2012). DOI: 10.1021/nl204201g
- D Kurouski, Vibrational Spectroscopy 91, 3 (2017). DOI: 10.1016/j.vibspec.2016.06.004
- A Dazzi, R Prazeres, F Glotin, and J M Ortega, OPTICS LETTERS 30, 2388 (2005). DOI: 10.1364/ol.30.002388
- A Dazzi, F Glotin, and R Carminati, Journal of Applied Physics 107, 124519 (2010). DOI: 10.1063/1.3429214
- J J Schwartz, D S Jakob, and A Centrone, Chemical Society Reviews 51, 5248 (2022). DOI: 10.1039/D2CS00095D
- C Marcott, M Lo, K Kjoller, C Prater, and I Noda, Applied Spectroscopy 65, 1145 (2011). DOI: 10.1366/11-06341
- A Dazzi, and C B Prater, Chemical Reviews 117, 5146 (2017). DOI: 10.1021/acs.chemrev.6b00448
- F Lu, M Jin, and M A Belkin, Nature Photonics 8, 307 (2014). DOI: 10.1038/nphoton.2013.373
- F S Ruggeri, B Mannini, R Schmid, M Vendruscolo, and T P J Knowles, Nature Communications 11, 2945 (2020). DOI: 10.1038/s41467-020-16728-1
- J Mathurin, E Pancani, A Deniset-Besseau, K Kjoller, C B Prater, R Gref, and A Dazzi, Analyst 143, 5940 (2018). DOI: 10.1039/C8AN01239C
- F Tarpoudi Baheri, T M Schutzius, D Poulikakos, and L D Poulikakos, Journal of Microscopy 00, 1 (2020). DOI: 10.1111/jmi.12890
- International Patent WO2020049053; United States Patent US 11,215,637 B2
- L Wang, H Wang, M Wagner, Y Yan, D S Jakob, X G Xu, Science Advance 3, e1700255 (2017). DOI: 10.1126/sciadv.1700255
- G Ramer, F S Ruggeri, A Levin, T P J Knowles, and A Centrone, ACS Nano 12, 6612 (2018). DOI: 10.1021/acsnano.8b01425
- A Miller, S Chia, Z Toprakcioglu, T Hakala, R Schmid, Y Feng, T Kartanas, A Kamada, M Vendruscolo, F S Ruggeri, and T P J Knowles, Science Advance 9, eabq3151 (2023). DOI: 10.1126/sciadv.abq3151
- A P Fellows, M T L Casford, and P B Davies, Biophysical Journal 119, 1474 (2020). DOI: 10.1016/j.bpj.2020.09.007

This information has been sourced, reviewed and adapted from materials provided by Bruker Nano Surfaces and Metrology.
For more information on this source, please visit Bruker Nano Surfaces and Metrology.