Sponsored by PerkinElmerReviewed by Olivia FrostJun 19 2024
Thermogravimetric analysis is a method where the weight of a sample is measured while it is concurrently being heated. This process can be performed under an array of purge gases that control material degradation.
Image Credit: Shutterstock/vchal
In most polymer studies, nitrogen or another inert gas, such as helium or argon, is used as a purge gas to ensure that the polymer undergoes pyrolysis instead of combustion.
In later stages, the purge gas can be switched to air or oxygen, which facilitates the quantification of carbon, whether from carbon black or carbon char produced during the polymer's pyrolysis. This is done by measuring the weight loss resulting from the combustion of carbon to form carbon dioxide. Any sample remaining after this process is typically classified as inorganic filler or "ash."
In this experiment, thermogravimetric analysis is used to decide core bits of information regarding an unknown polymer.
Experimental
A 13.8 mg sample of an unknown polymer was put into a ceramic crucible (N5200040) with no additional sample treatment or preparation. The sample was heated using the below table under a nitrogen purge (50 mL/minute):
- Hold at 25 ℃ for 1 minute
- Heat from 25 to 800 ℃ at 10 ℃/minute
- Hold at 800 ℃ for 5 minutes
- Switch the purge gas to air (50 mL/minute)
- Hold at 800 ℃ for 10 minutes
In thermogravimetric analysis, every step in the heating process has a designated purpose. The steps of the above heating program can be explained in a similar manner, as shown in Table 1.
Table 1. Description of the purpose of each step of the thermal program. Source: PerkinElmer
| Step |
Purpose |
| 1 |
Allow the sample temperature and purge gas to equilibrate |
| 2-3 |
Quantify free and bound water and other volatiles, plasticizers and other additives, polymer degradation, initial decarboxylation of calcium carbonate |
| 4 |
Change the purge gas such that further measurement is carried out under an oxidative atmosphere |
| 5 |
Determination of carbon char, carbon black and residual filler/ash content |

Figure 1. PerkinElmer Pyris™ TGA 9 Thermogravimetric Analyzer. Image Credit: PerkinElmer
The above heating program was performed using the PerkinElmer TGA 9, as seen in Figure 1.
Results
The thermogram of the unknown polymer is seen in Figure 2.
The first weight loss, between 100 ℃ and 220 ℃, is a result of bound water evolution (1.16 %). The subsequent degradation, with an onset temperature of 400 ℃, is the pyrolysis of the polymer (64.23 %). The last degradation that arises under a nitrogen atmosphere is the decarboxylation of calcium carbonate, which produces calcium oxide (5.423 %)
Stoichiometric calculations can be utilized to determine the total quantity of calcium carbonate in the material using this equation:
CaCO3 → CaO + CO2
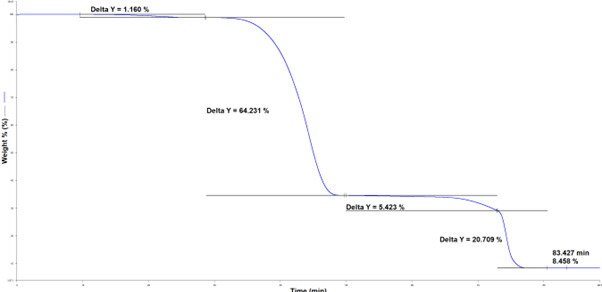
Figure 2. TGA data obtained from analysis of an unknown polymer material. Image Credit: PerkinElmer
In this experiment, CaCO3 shows a molecular weight of 100.1 amu, while the degradation products, CaO and CO2, show molecular weights of 56.1 and 44.0 amu, respectively.
Consequently, it can be determined that the polymer experiences a 5.42 % loss due to decarboxylation, indicated by the removal of CO2. This loss signifies that the initial total weight percentage of CaCO3 in the sample was 12.33 % (equivalent to 1.7 mg). Consequently, the remaining weight percentage of CaO is 6.91 %.
This analysis also facilitates the computation of any additional inorganic fillers by subtracting the calculated weight of CaO from the final mass recorded at the conclusion of the TGA run, which is 8.46 %. The result is a residual inorganic filler weight percentage of 1.55% (or 0.21 mg).
Lastly, the degradation observed after switching to air is due to carbon combustion, indicating that the carbon content of this material is 20.71 %. This carbon could originate from two possible sources: either carbon black present in the sample or carbon produced during the pyrolysis of the sample.
Summary
The PerkinElmer TGA 9 thermogravimetric analyzer offers a powerful method for quantifying a variety of components in complex material formulations. In combination with Pyris software, users can calculate mass losses and streamline materials research quickly and easily.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.