Decarbonization is a global key market objective, with the goal of achieving it by 2050 and creating more efficient energy for a sustainable planet. Decarbonization offers a solution to issues created by fossil fuels, such as gas emissions that contaminate the atmosphere with SOx, NOx, and CO2, as well as issues with supply and prices.

Image Credit: AIMPLAS
Today's energy situation has underscored the urgent need to find alternatives to gas and oil to ensure greater energy independence. A complete transition to hydrogen will play a crucial role in decarbonizing all sectors of the economy, paving the way for a comprehensive hydrogen roadmap.
Identifying the required priorities and resources, the key challenges in developing renewable hydrogen, and possible methods for overcoming those issues can make the deployment of this energy type in Spain possible.
This document aligns with the Annual Strategy of Sustainable Growth of 2021 published by the European Commission.
What is Hydrogen?
Hydrogen is the most abundant and simple element in existence. It has the potential to provide energy to all manner of transport or buildings. Hydrogen is not a primary energy source but an energy carrier. It is produced from a primary energy resource (ideally renewable) and serves as the transportation and storage of that energy. When electricity comes from a renewable source, it is known as green hydrogen.
According to the International Agency of Energy (IAE), 70 million tons of hydrogen are consumed globally. This is mostly as a reactive for a variety of processes in the chemistry sector, such as producing ammonia and hydrocracking and desulfuration of fuels. Around 80% of the global demand can be attributed to these uses.
Once produced, hydrogen’s chemical energy can transform into various forms of energy, giving place to the concept of routes of valuation Power-to-X. Hydrogen can transform into electricity (power-to-power) or methane, or it can be injected into natural gas (power-to-gas), serving as a primary substance for the chemical industry (power-to-industry), transforming into fuel, methanol (power-to-fuels), or for mobility (power-to-mobility).
Through these various focuses, hydrogen can integrate renewable energies on a large scale and create energy, performing as a shock absorber for the escalation in system flexibility and assisting with the decarbonization of all the primary economic sectors.
The EU has estimated that by 2050, hydrogen will encompass a 14% energy mix, gain sufficient maturity, and be released on a large scale, surpassing the challenges of technology in regard to cost, production, or limited distribution through the current gas network.
What Advantages Does Hydrogen Have?
Using hydrogen for energy transition provides numerous advantages as hydrogen is unique to other fossil fuels:
- It does not produce CO2 emissions
- It has a high density of energy per unit of mass
- It needs a low activation energy
- It is highly volatile
- It is not toxic
- It has a high inferior limit of inflammability and detonation and a high temperature for spontaneous combustion
- It is very secure in open spaces
Types of Hydrogen Production:
There are three primary sources of obtaining hydrogen:
- Non-renewable fossil resources, including natural gas, oil, and carbon (such as uranium).
- Renewable resources include solar energy, wind, hydraulic, geothermal, and biomass.
- Nuclear energy– depends on the resource type and the process used to obtain it, the resulting hydrogen can be classified into various colors.
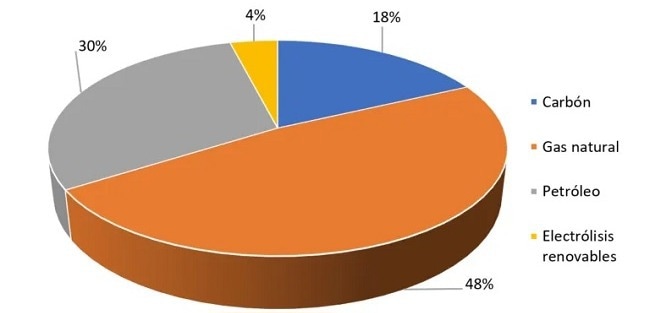
Figure. Hydrogen as an energy vector. Source: Asociación española del hidrógeno, AeH2. Image Credit: AIMPLAS
Based on Fossil Resources
95% of hydrogen is obtained through other combustibles, the most significant source being natural gas. This type generates large amounts of CO2, but there are ways to recapture it.
The chemical processes for obtaining hydrogen based on natural gas or carbon are:
- Reforming the vapor: gray hydrogen
- Partial oxidation: gray hydrogen
- Reforming auto thermal: gray hydrogen
- Vaporization: blue hydrogen
- Pyrolysis: turquoise hydrogen
- Reforming the vapor/ vaporization by capturing CO2: blue hydrogen
Renewable Resources
Only about 4% of hydrogen is obtained from renewable resources. Testing of an impulse without precedent that can establish the basis for its enormous potential, such as clean energy, is in progress. The processes for obtaining renewable hydrogen are:
- Electrolysis: with the electricity of the yellow hydrogen network, renewable (wind, solar, hydraulic, biomass) green hydrogen.
- Reforming the vapor: renewable (wind, solar, hydraulic, biomass) green energy.
- The vaporization of biomass: renewable (wind, solar, hydraulic, biomass) green hydrogen.
Nuclear Energy
The production of hydrogen through nuclear energy provides the opportunity to drastically recapture carbon emissions while boosting the profitability of the nuclear-electronic sector. The power of nuclear reactors can be combined with a hydrogen production plant to arrive at a more efficient mode of energy and hydrogen in a co-generation system. Hydrogen produced by nuclear energy can be obtained through:
- Electrolysis: purple hydrogen
- Thermochemical cycles: purple hydrogen
Hydrogen Storage
Effective hydrogen storage is essential for developing an economy based on it. This is one of the critical challenges, as hydrogen is difficult to store. Similar to gas, it contains 7% density in the air. In its liquid state, it is -253 ºC with a 7% density in water. The storage system and conditions must vary depending on the intended use.
It is possible to use stable storage systems, which can be used in residential areas for decentralized generation of electricity or industrial uses, for example. In these cases, the hydrogen storage systems have fewer limitations regarding concerns such as surface occupation, weight, and volume, or necessity for peripheral systems.
There are much greater restrictions in terms of weight and volume for automotive uses. Minimum limits on the quality of hydrogen are increasing, and they need to be managed for the vehicles to achieve equivalency with conventional vehicles, as established by the Department of Energy of the EU.
There are also barriers to operating conditions and hydrogen supply kinetics regarding their use in combination with combustible batteries. This makes it necessary to build technology that can overcome these barriers by improving isolation or weight lightening, for example.
It is worth noting that the characteristics of hydrogen also make security a key conditioning factor when selecting a hydrogen storage system. Several possible options fulfill the requirements in different situations. These systems of storage include:
- Gas pressure
- Liquid (cryogenic storage)
- Metal hydrides
- Porous polymers such as active carbon, graphite, zeolites, MOF, COF, etc.
- In the form of chemical compounds (NH3, toluene, etc)
- In glass microspheres
Only the first three options are reliable enough to be presented to the market. There is much room for an open investigation.
Projects at AIMPLAS
AIMPLAS answers the challenges of generating and using hydrogen as a renewable fuel. It works on various projects to meet the industry's needs and improve the chances of applying innovative results.
The objectives pursued are based on the following:
- Evaluation of alternative energy vectors to those of fossil origin from waste.
- Selective hydrogen production through catalyst-assisted thermochemical treatment processes.
- Design and modification of polymeric and nanoporous materials for the diverse, effective storage of energy vectors, improving weight and permeability.
- Viability studies for developments on generating hydrogen through valorizing the complex plastic waste.

This information has been sourced, reviewed and adapted from materials provided by AIMPLAS.
For more information on this source, please visit AIMPLAS.